Bioactive glass in tissue engineering
- PMID: 21421084
- PMCID: PMC3085647
- DOI: 10.1016/j.actbio.2011.03.016
Bioactive glass in tissue engineering
Abstract
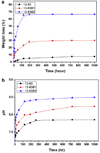
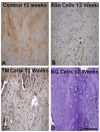
This review focuses on recent advances in the development and use of bioactive glass for tissue engineering applications. Despite its inherent brittleness, bioactive glass has several appealing characteristics as a scaffold material for bone tissue engineering. New bioactive glasses based on borate and borosilicate compositions have shown the ability to enhance new bone formation when compared to silicate bioactive glass. Borate-based bioactive glasses also have controllable degradation rates, so the degradation of the bioactive glass implant can be more closely matched to the rate of new bone formation. Bioactive glasses can be doped with trace quantities of elements such as Cu, Zn and Sr, which are known to be beneficial for healthy bone growth. In addition to the new bioactive glasses, recent advances in biomaterials processing have resulted in the creation of scaffold architectures with a range of mechanical properties suitable for the substitution of loaded as well as non-loaded bone. While bioactive glass has been extensively investigated for bone repair, there has been relatively little research on the application of bioactive glass to the repair of soft tissues. However, recent work has shown the ability of bioactive glass to promote angiogenesis, which is critical to numerous applications in tissue regeneration, such as neovascularization for bone regeneration and the healing of soft tissue wounds. Bioactive glass has also been shown to enhance neocartilage formation during in vitro culture of chondrocyte-seeded hydrogels, and to serve as a subchondral substrate for tissue-engineered osteochondral constructs. Methods used to manipulate the structure and performance of bioactive glass in these tissue engineering applications are analyzed.
Copyright © 2011 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Figures


















References
-
- Nerem RM. Cellular engineering. Ann Biomed Eng. 1991;19:529–545. - PubMed
-
- Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. - PubMed
-
- Elisseeff J, McIntosh W, Fu K, Blunk R, Langer R. Controlled-release of IGF-I and TGF-β 1 in a photopolymerizing hydrogel for cartilage tissue engineering. J Orthop Res. 2001;19:1098–1104. - PubMed
-
- Babensee JE, McIntire LV, Mikos AG. Growth factor delivery for tissue engineering. Pharmaceut Res. 2000;17:497–504. - PubMed
-
- Shea LD, Smiley E, Bonadio J, Mooney DJ. DNA delivery from polymer matrices for tissue engineering. Nat Biotechnol. 1999;17:551–554. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

