Structural comparisons of arachidonic acid-induced radicals formed by prostaglandin H synthase-1 and -2
- PMID: 21421123
- PMCID: PMC3073652
- DOI: 10.1016/j.jinorgbio.2010.11.012
Structural comparisons of arachidonic acid-induced radicals formed by prostaglandin H synthase-1 and -2
Abstract
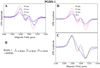
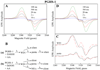
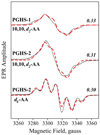
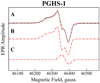
Cyclooxygenase catalysis by prostaglandin H synthase (PGHS)-1 and -2 involves reaction of a peroxide-induced Tyr385 radical with arachidonic acid (AA) to form an AA radical that reacts with O(2). The potential for isomeric AA radicals and formation of an alternate tyrosyl radical at Tyr504 complicate analysis of radical intermediates. We compared the EPR spectra of PGHS-1 and -2 reacted with peroxide and AA or specifically deuterated AA in anaerobic, single-turnover experiments. With peroxide-treated PGHS-2, the carbon-centered radical observed after AA addition was consistently a pentadienyl radical; a variable wide-singlet (WS) contribution from mixture of Tyr385 and Tyr504 radicals was also present. Analogous reactions with PGHS-1 produced EPR signals consistent with varying proportions of pentadienyl and tyrosyl radicals, and two additional EPR signals. One, insensitive to oxygen exposure, is the narrow singlet tyrosyl radical with clear hyperfine features found previously in inhibitor-pretreated PGHS-1. The second type of EPR signal is a narrow singlet lacking detailed hyperfine features that disappeared upon oxygen exposure. This signal was previously ascribed to an allyl radical, but high field EPR analysis indicated that ~90% of the signal originates from a novel tyrosyl radical, with a small contribution from a carbon-centered species. The radical kinetics could be resolved by global analysis of EPR spectra of samples trapped at various times during anaerobic reaction of PGHS-1 with a mixture of peroxide and AA. The improved understanding of the dynamics of AA and tyrosyl radicals in PGHS-1 and -2 will be useful for elucidating details of the cyclooxygenase mechanism, particularly the H-transfer between tyrosyl radical and AA.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Structural characterization of arachidonyl radicals formed by prostaglandin H synthase-2 and prostaglandin H synthase-1 reconstituted with mangano protoporphyrin IX.J Biol Chem. 1998 Feb 13;273(7):3888-94. doi: 10.1074/jbc.273.7.3888. J Biol Chem. 1998. PMID: 9461572
-
Cyclooxygenase reaction mechanism of PGHS--evidence for a reversible transition between a pentadienyl radical and a new tyrosyl radical by nitric oxide trapping.J Inorg Biochem. 2011 Mar;105(3):356-65. doi: 10.1016/j.jinorgbio.2010.11.013. J Inorg Biochem. 2011. PMID: 21403766 Free PMC article.
-
Structural characterization of arachidonyl radicals formed by aspirin-treated prostaglandin H synthase-2.J Biol Chem. 2002 Oct 11;277(41):38311-21. doi: 10.1074/jbc.M206961200. Epub 2002 Aug 6. J Biol Chem. 2002. PMID: 12167656
-
Free radical-dependent inhibition of prostaglandin endoperoxide H Synthase-2 by nitro-arachidonic acid.Free Radic Biol Med. 2019 Nov 20;144:176-182. doi: 10.1016/j.freeradbiomed.2019.03.022. Epub 2019 Mar 25. Free Radic Biol Med. 2019. PMID: 30922958 Review.
-
Prostaglandin H synthase: resolved and unresolved mechanistic issues.Arch Biochem Biophys. 2010 Jan 1;493(1):103-24. doi: 10.1016/j.abb.2009.08.019. Epub 2009 Sep 1. Arch Biochem Biophys. 2010. PMID: 19728984 Free PMC article. Review.
Cited by
-
Dynamics of Radical Intermediates in Prostaglandin H Synthase-1 Cyclooxygenase Reactions is Modulated by Multiple Factors.Protein Pept Lett. 2016;23(11):1013-1023. doi: 10.2174/0929866523666161007151812. Protein Pept Lett. 2016. PMID: 27748183 Free PMC article.
References
-
- Smith WL, Garavito RM, DeWitt DL. J. Biol. Chem. 1996;271:33157–33160. - PubMed
-
- Herschman HR. Biochim. Biophys. Acta. 1996;1299:125–140. - PubMed
-
- Picot D, Loll PJ, Garavito RM. Nature. 1994;367:243–249. - PubMed
-
- Kurumbail RG, Stevens AM, Gierse JK, McDonald JJ, Stegeman RA, Pak JY, Gildehaus D, Miyashiro JM, Penning TD, Seibert K, Isakson PC, Stallings WC. Nature. 1996;384:644–648. - PubMed
-
- Luong C, Miller A, Barnett J, Chow J, Ramesha C, Browner MF. Nat. Struct. Biol. 1996;3:927–933. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

