Autonomic remodeling in the left atrium and pulmonary veins in heart failure: creation of a dynamic substrate for atrial fibrillation
- PMID: 21421805
- PMCID: PMC3607326
- DOI: 10.1161/CIRCEP.110.959650
Autonomic remodeling in the left atrium and pulmonary veins in heart failure: creation of a dynamic substrate for atrial fibrillation
Abstract
Background: Atrial fibrillation (AF) is commonly associated with congestive heart failure (CHF). The autonomic nervous system is involved in the pathogenesis of both AF and CHF. We examined the role of autonomic remodeling in contributing to AF substrate in CHF.
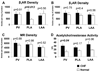
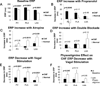
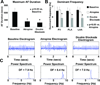
Methods and results: Electrophysiological mapping was performed in the pulmonary veins and left atrium in 38 rapid ventricular-paced dogs (CHF group) and 39 control dogs under the following conditions: vagal stimulation, isoproterenol infusion, β-adrenergic blockade, acetylcholinesterase (AChE) inhibition (physostigmine), parasympathetic blockade, and double autonomic blockade. Explanted atria were examined for nerve density/distribution, muscarinic receptor and β-adrenergic receptor densities, and AChE activity. In CHF dogs, there was an increase in nerve bundle size, parasympathetic fibers/bundle, and density of sympathetic fibrils and cardiac ganglia, all preferentially in the posterior left atrium/pulmonary veins. Sympathetic hyperinnervation was accompanied by increases in β(1)-adrenergic receptor R density and in sympathetic effect on effective refractory periods and activation direction. β-Adrenergic blockade slowed AF dominant frequency. Parasympathetic remodeling was more complex, resulting in increased AChE activity, unchanged muscarinic receptor density, unchanged parasympathetic effect on activation direction and decreased effect of vagal stimulation on effective refractory period (restored by AChE inhibition). Parasympathetic blockade markedly decreased AF duration.
Conclusions: In this heart failure model, autonomic and electrophysiological remodeling occurs, involving the posterior left atrium and pulmonary veins. Despite synaptic compensation, parasympathetic hyperinnervation contributes significantly to AF maintenance. Parasympathetic and/or sympathetic signaling may be possible therapeutic targets for AF in CHF.
Conflict of interest statement
Figures



References
-
- Ehrlich JR, Nattel S, Hohnloser SH. Atrial fibrillation and congestive heart failure: specific considerations at the intersection of two common and important cardiac disease sets. J Cardiovasc Electrophysiol. 2002;13:399–405. - PubMed
-
- Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, D'Agostino RB, Murabito JM, Kannel WB, Benjamin EJ. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107:2920–2925. - PubMed
-
- Li D, Melnyk P, Feng J, Wang Z, Petrecca K, Shrier A, Nattel S. Effects of experimental heart failure on atrial cellular and ionic electrophysiology. Circulation. 2000;101:2631–2638. - PubMed
-
- Li D, Fareh S, Leung TK, Nattel S. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation. 1999;100:87–95. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

