Inducible costimulator controls migration of T cells to the lungs via down-regulation of CCR7 and CD62L
- PMID: 21421907
- PMCID: PMC3208611
- DOI: 10.1165/rcmb.2010-0466OC
Inducible costimulator controls migration of T cells to the lungs via down-regulation of CCR7 and CD62L
Abstract
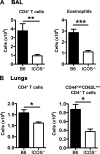
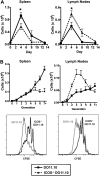
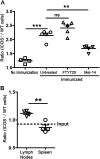
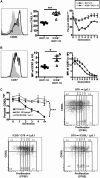
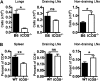
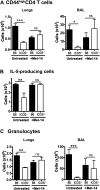
We and others reported that inducible costimulator-deficient (ICOS(-/-)) mice manifest a defect in Th2-mediated airway inflammation, which was attributed to reduced Th2 differentiation in the absence of ICOS signaling. Interestingly, the number of CD4 T cells present in the airways and lungs after sensitization and challenge is significantly reduced in ICOS(-/-) mice. We now show that this reduction is not attributable simply to a reduced proliferation of ICOS(-/-) cells, because significantly more ICOS(-/-) than wild-type activated CD4 T cells are present in the lymph nodes, suggesting that more ICOS(-/-) CD4 T cells than wild-type CD4 T cells migrated into the lymph nodes. Further investigation revealed that activated ICOS(-/-) CD4 T cells express higher concentrations of the lymph node homing receptors, CCR7 and CD62L, than do wild-type CD4 T cells, leading to a preferential return of ICOS(-/-) cells to the nondraining lymph nodes rather than the lungs. Blocking reentry into the lymph nodes after the initiation of Th2-mediated airway inflammation equalized the levels of CD4 and granulocyte infiltration in the lungs of wild-type and ICOS(-/-) mice. Our results demonstrate that in wild-type CD4 T cells, co-stimulation with ICOS promotes the down-regulation of CCR7 and CD62L after activation, leading to a reduced return of activated CD4 T cells to the lymph nodes and a more efficient entry into the lungs.
Figures
Similar articles
-
Inducible costimulator regulates Th2-mediated inflammation, but not Th2 differentiation, in a model of allergic airway disease.J Immunol. 2001 Aug 15;167(4):1996-2003. doi: 10.4049/jimmunol.167.4.1996. J Immunol. 2001. PMID: 11489981
-
CD4+ICOS+ T lymphocytes inhibit T cell activation 'in vitro' and attenuate autoimmune encephalitis 'in vivo'.Int Immunol. 2008 Apr;20(4):577-89. doi: 10.1093/intimm/dxn016. Epub 2008 Feb 28. Int Immunol. 2008. PMID: 18310064
-
ICOS costimulation expands Th2 immunity by augmenting migration of lymphocytes to draining lymph nodes.J Immunol. 2008 Jul 15;181(2):1019-24. doi: 10.4049/jimmunol.181.2.1019. J Immunol. 2008. PMID: 18606653 Free PMC article.
-
The TLR4-TRIF pathway can protect against the development of experimental allergic asthma.Immunology. 2017 Sep;152(1):138-149. doi: 10.1111/imm.12755. Epub 2017 Jun 20. Immunology. 2017. PMID: 28502093 Free PMC article.
-
Regulation of CD4 T cell activation and effector function by inducible costimulator (ICOS).Curr Opin Immunol. 2010 Jun;22(3):326-32. doi: 10.1016/j.coi.2010.01.001. Epub 2010 Jan 29. Curr Opin Immunol. 2010. PMID: 20116985 Review.
Cited by
-
Down-Regulation of CD62L Shedding in T Cells by CD39+ Regulatory T Cells Leads to Defective Sensitization in Contact Hypersensitivity Reactions.J Invest Dermatol. 2017 Jan;137(1):106-114. doi: 10.1016/j.jid.2016.08.023. Epub 2016 Sep 10. J Invest Dermatol. 2017. PMID: 27623510 Free PMC article.
-
ICOS-expressing lymphocytes promote resolution of CD8-mediated lung injury in a mouse model of lung rejection.PLoS One. 2013 Aug 13;8(8):e72955. doi: 10.1371/journal.pone.0072955. eCollection 2013. PLoS One. 2013. PMID: 23967339 Free PMC article.
-
ICOS+ Tregs: A Functional Subset of Tregs in Immune Diseases.Front Immunol. 2020 Aug 28;11:2104. doi: 10.3389/fimmu.2020.02104. eCollection 2020. Front Immunol. 2020. PMID: 32983168 Free PMC article. Review.
-
Retrogenic ICOS Expression Increases Differentiation of KLRG-1hiCD127loCD8+ T Cells during Listeria Infection and Diminishes Recall Responses.J Immunol. 2016 Feb 1;196(3):1000-12. doi: 10.4049/jimmunol.1500218. Epub 2016 Jan 4. J Immunol. 2016. PMID: 26729800 Free PMC article.
-
Regulatory T cells and the control of the allergic response.J Allergy (Cairo). 2012;2012:948901. doi: 10.1155/2012/948901. Epub 2012 Sep 29. J Allergy (Cairo). 2012. PMID: 23056063 Free PMC article.
References
-
- Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB. Predominant Th2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med 1992;326:298–304 - PubMed
-
- Woodland DL, Hogan RJ, Zhong W. Cellular immunity and memory to respiratory virus infections. Immunol Res 2001;24:53–67 - PubMed
-
- Swain SL, Dutton RW, Woodland DL. T cell responses to influenza virus infection: effector and memory cells. Viral Immunol 2004;17:197–209 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

