Basement membranes: cell scaffoldings and signaling platforms
- PMID: 21421915
- PMCID: PMC3039528
- DOI: 10.1101/cshperspect.a004911
Basement membranes: cell scaffoldings and signaling platforms
Abstract
Basement membranes are widely distributed extracellular matrices that coat the basal aspect of epithelial and endothelial cells and surround muscle, fat, and Schwann cells. These extracellular matrices, first expressed in early embryogenesis, are self-assembled on competent cell surfaces through binding interactions among laminins, type IV collagens, nidogens, and proteoglycans. They form stabilizing extensions of the plasma membrane that provide cell adhesion and that act as solid-phase agonists. Basement membranes play a role in tissue and organ morphogenesis and help maintain function in the adult. Mutations adversely affecting expression of the different structural components are associated with developmental arrest at different stages as well as postnatal diseases of muscle, nerve, brain, eye, skin, vasculature, and kidney.
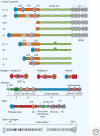
Figures


References
-
- Abrahamson DR 1987. Structure and development of the glomerular capillary wall and basement membrane. Am J Physiol 253: F783–F794 - PubMed
-
- Aumailley M, Bruckner-Tuderman L, Carter WG, Deutzmann R, Edgar D, Ekblom P, Engel J, Engvall E, Hohenester E, Jones JC, et al. 2005. A simplified laminin nomenclature. Matrix Biol 24: 326–332 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
