Integrin structure, activation, and interactions
- PMID: 21421922
- PMCID: PMC3039929
- DOI: 10.1101/cshperspect.a004994
Integrin structure, activation, and interactions
Abstract
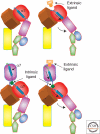
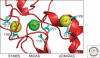
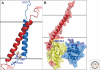
Integrins are large, membrane-spanning, heterodimeric proteins that are essential for a metazoan existence. All members of the integrin family adopt a shape that resembles a large "head" on two "legs," with the head containing the sites for ligand binding and subunit association. Most of the receptor dimer is extracellular, but both subunits traverse the plasma membrane and terminate in short cytoplasmic domains. These domains initiate the assembly of large signaling complexes and thereby bridge the extracellular matrix to the intracellular cytoskeleton. To allow cells to sample and respond to a dynamic pericellular environment, integrins have evolved a highly responsive receptor activation mechanism that is regulated primarily by changes in tertiary and quaternary structure. This review summarizes recent progress in the structural and molecular functional studies of this important class of adhesion receptor.
Figures
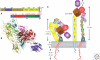
References
-
- Alon R, Dustin ML 2007. Force as a facilitator of integrin conformational changes during leukocyte arrest on blood vessels and antigen-presenting cells. Immunity 26: 17–27 - PubMed
-
- Alonso JL, Essafi M, Xiong JP, Stehle T, Arnaout MA 2002. Does the integrin αA domain act as a ligand for its βA domain? Curr Biol 12: R340–R342 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
