Conserved oligomeric Golgi complex specifically regulates the maintenance of Golgi glycosylation machinery
- PMID: 21421995
- PMCID: PMC3219415
- DOI: 10.1093/glycob/cwr028
Conserved oligomeric Golgi complex specifically regulates the maintenance of Golgi glycosylation machinery
Abstract
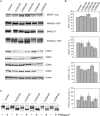
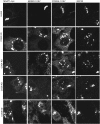
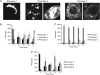
Cell surface lectin staining, examination of Golgi glycosyltransferases stability and localization, and matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) analysis were employed to investigate conserved oligomeric Golgi (COG)-dependent glycosylation defects in HeLa cells. Both Griffonia simplicifolia lectin-II and Galanthus nivalus lectins were specifically bound to the plasma membrane glycoconjugates of COG-depleted cells, indicating defects in activity of medial- and trans-Golgi-localized enzymes. In response to siRNA-induced depletion of COG complex subunits, several key components of Golgi glycosylation machinery, including MAN2A1, MGAT1, B4GALT1 and ST6GAL1, were severely mislocalized. MALDI-TOF analysis of total N-linked glycoconjugates indicated a decrease in the relative amount of sialylated glycans in both COG3 KD and COG4 KD cells. In agreement to a proposed role of the COG complex in retrograde membrane trafficking, all types of COG-depleted HeLa cells were deficient in the Brefeldin A- and Sar1 DN-induced redistribution of Golgi resident glycosyltransferases to the endoplasmic reticulum. The retrograde trafficking of medial- and trans-Golgi-localized glycosylation enzymes was affected to a larger extent, strongly indicating that the COG complex regulates the intra-Golgi protein movement. COG complex-deficient cells were not defective in Golgi re-assembly after the Brefeldin A washout, confirming specificity in the retrograde trafficking block. The lobe B COG subcomplex subunits COG6 and COG8 were localized on trafficking intermediates that carry Golgi glycosyltransferases, indicating that the COG complex is directly involved in trafficking and maintenance of Golgi glycosylation machinery.
© The Author 2011. Published by Oxford University Press. All rights reserved.
Figures



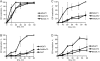


References
-
- Aoki D, Lee N, Yamaguchi N, Dubois C, Fukuda MN. Golgi retention of a trans-Golgi membrane protein, galactosyltransferase, requires cysteine and histidine residues within the membrane-anchoring domain. Proc Natl Acad Sci USA. 1992;89:4319–4323. doi:10.1073/pnas.89.10.4319. - DOI - PMC - PubMed
-
- Bonifacino JS, Glick BS. The mechanisms of vesicle budding and fusion. Cell. 2004;116:153–166. doi:10.1016/S0092-8674(03)01079-1. - DOI - PubMed
-
- Bruinsma P, Spelbrink RG, Nothwehr SF. Retrograde transport of the mannosyltransferase Och1p to the early Golgi requires a component of the COG transport complex. J Biol Chem. 2004;279:39814–39823. doi:10.1074/jbc.M405500200. - DOI - PubMed
-
- Chen R, Jiang X, Sun D, Han G, Wang F, Ye M, Wang L, Zou H. Glycoproteomics analysis of human liver tissue by combination of multiple enzyme digestion and hydrazide chemistry. J Proteome Res. 2009;8:651–661. doi:10.1021/pr8008012. - DOI - PubMed
-
- Foulquier F. COG defects, birth and rise! Biochim Biophys Acta. 2009;1792:896–902. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

