SpyA, a C3-like ADP-ribosyltransferase, contributes to virulence in a mouse subcutaneous model of Streptococcus pyogenes infection
- PMID: 21422178
- PMCID: PMC3125853
- DOI: 10.1128/IAI.01191-10
SpyA, a C3-like ADP-ribosyltransferase, contributes to virulence in a mouse subcutaneous model of Streptococcus pyogenes infection
Abstract
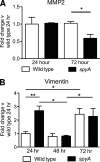
Streptococcus pyogenes is an important human pathogen with an expansive repertoire of verified and putative virulence factors. Here we demonstrate that a mutant deficient in the production of the streptococcal ADP-ribosyltransferase SpyA generates lesions of reduced size in a subcutaneous mouse infection model. At early stages of infection, when the difference in lesion size is first established, inflamed tissue isolated from lesions of mice infected with spyA mutant bacteria has higher levels of mRNA encoding the chemokines CXCL1 and CCL2 than does tissue isolated from mice infected with wild-type bacteria. In addition, at these early times, the mRNA levels for the gene encoding the intermediate filament vimentin are higher in the mutant-infected tissue. As wound resolution progresses, mRNA levels of the gene encoding matrix metallopeptidase 2 are lower in mutant-infected tissue. Furthermore, we demonstrate that the spyA mutant is internalized more efficiently than wild-type bacteria by HeLa cells. We conclude that SpyA contributes to streptococcal pathogenesis in the mouse subcutaneous infection model. Our observations suggest that the presence of SpyA delays wound healing in this model.
Figures
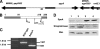
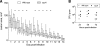
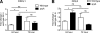

Similar articles
-
Molecular and biological characterization of Streptococcal SpyA-mediated ADP-ribosylation of intermediate filament protein vimentin.J Biol Chem. 2012 Jun 15;287(25):21481-91. doi: 10.1074/jbc.M112.370791. Epub 2012 May 1. J Biol Chem. 2012. PMID: 22549780 Free PMC article.
-
SpyA is a membrane-bound ADP-ribosyltransferase of Streptococcus pyogenes which modifies a streptococcal peptide, SpyB.Mol Microbiol. 2012 Mar;83(5):936-52. doi: 10.1111/j.1365-2958.2012.07979.x. Epub 2012 Jan 30. Mol Microbiol. 2012. PMID: 22288436 Free PMC article.
-
Identification of SpyA, a novel ADP-ribosyltransferase of Streptococcus pyogenes.Mol Microbiol. 2004 Oct;54(1):89-98. doi: 10.1111/j.1365-2958.2004.04262.x. Mol Microbiol. 2004. PMID: 15458407
-
Streptococcal toxins: role in pathogenesis and disease.Cell Microbiol. 2015 Dec;17(12):1721-41. doi: 10.1111/cmi.12531. Epub 2015 Nov 17. Cell Microbiol. 2015. PMID: 26433203 Review.
-
[Persistence of Streptococcus pyogenes].Zh Mikrobiol Epidemiol Immunobiol. 2009 May-Jun;(3):104-9. Zh Mikrobiol Epidemiol Immunobiol. 2009. PMID: 19621829 Review. Russian.
Cited by
-
Group A Streptococcus encounters with host macrophages.Future Microbiol. 2018 Jan;13(1):119-134. doi: 10.2217/fmb-2017-0142. Epub 2017 Dec 11. Future Microbiol. 2018. PMID: 29226710 Free PMC article. Review.
-
Novel bacterial ADP-ribosylating toxins: structure and function.Nat Rev Microbiol. 2014 Sep;12(9):599-611. doi: 10.1038/nrmicro3310. Epub 2014 Jul 14. Nat Rev Microbiol. 2014. PMID: 25023120 Free PMC article. Review.
-
Playing With Fire: Proinflammatory Virulence Mechanisms of Group A Streptococcus.Front Cell Infect Microbiol. 2021 Jul 6;11:704099. doi: 10.3389/fcimb.2021.704099. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34295841 Free PMC article. Review.
-
SpyB, a Small Heme-Binding Protein, Affects the Composition of the Cell Wall in Streptococcus pyogenes.Front Cell Infect Microbiol. 2016 Oct 13;6:126. doi: 10.3389/fcimb.2016.00126. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 27790410 Free PMC article.
-
The Role of Streptococcal and Staphylococcal Exotoxins and Proteases in Human Necrotizing Soft Tissue Infections.Toxins (Basel). 2019 Jun 11;11(6):332. doi: 10.3390/toxins11060332. Toxins (Basel). 2019. PMID: 31212697 Free PMC article. Review.
References
-
- Aepfelbacher M., Essler M., Huber E., Sugai M., Weber P. C. 1997. Bacterial toxins block endothelial wound repair. Evidence that Rho GTPases control cytoskeletal rearrangements in migrating endothelial cells. Arterioscler. Thromb. Vasc. Biol. 17:1623–1629 - PubMed
-
- Beneš P., et al. 2006. Role of vimentin in regulation of monocyte/macrophage differentiation. Differentiation 74:265–276 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

