MyD88 signaling is required for efficient innate and adaptive immune responses to Paracoccidioides brasiliensis infection
- PMID: 21422180
- PMCID: PMC3125857
- DOI: 10.1128/IAI.00375-10
MyD88 signaling is required for efficient innate and adaptive immune responses to Paracoccidioides brasiliensis infection
Abstract
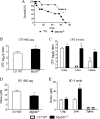
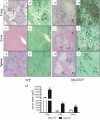
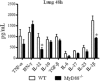
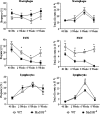
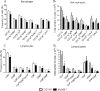
The mechanisms that govern the initial interaction between Paracoccidioides brasiliensis, a primary dimorphic fungal pathogen, and cells of the innate immunity need to be clarified. Our previous studies showed that Toll-like receptor 2 (TLR2) and TLR4 regulate the initial interaction of fungal cells with macrophages and the pattern of adaptive immunity that further develops. The aim of the present investigation was to assess the role of MyD88, an adaptor molecule used by TLRs to activate genes of the inflammatory response in pulmonary paracoccidioidomycosis. Studies were performed with normal and MyD88(-/-) C57BL/6 mice intratracheally infected with P. brasiliensis yeast cells. MyD88(-/-) macrophages displayed impaired interaction with fungal yeast cells and produced low levels of IL-12, MCP-1, and nitric oxide, thus allowing increased fungal growth. Compared with wild-type (WT) mice, MyD88(-/-) mice developed a more severe infection of the lungs and had marked dissemination of fungal cells to the liver and spleen. MyD88(-/-) mice presented low levels of Th1, Th2, and Th17 cytokines, suppressed lymphoproliferation, and impaired influx of inflammatory cells to the lungs, and this group of cells comprised lower numbers of neutrophils, activated macrophages, and T cells. Nonorganized, coalescent granulomas, which contained high numbers of fungal cells, characterized the severe lesions of MyD88(-/-) mice; the lesions replaced extensive areas of several organs. Therefore, MyD88(-/-) mice were unable to control fungal growth and showed a significantly decreased survival time. In conclusion, our findings demonstrate that MyD88 signaling is important in the activation of fungicidal mechanisms and the induction of protective innate and adaptive immune responses against P. brasiliensis.
Figures


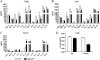

Similar articles
-
Toll-like receptor 4 signaling leads to severe fungal infection associated with enhanced proinflammatory immunity and impaired expansion of regulatory T cells.Infect Immun. 2010 Mar;78(3):1078-88. doi: 10.1128/IAI.01198-09. Epub 2009 Dec 14. Infect Immun. 2010. PMID: 20008536 Free PMC article.
-
MyD88 is dispensable for resistance to Paracoccidioides brasiliensis in a murine model of blood-borne disseminated infection.FEMS Immunol Med Microbiol. 2008 Dec;54(3):365-74. doi: 10.1111/j.1574-695X.2008.00487.x. FEMS Immunol Med Microbiol. 2008. PMID: 19049649
-
Absence of interleukin-4 determines less severe pulmonary paracoccidioidomycosis associated with impaired Th2 response.Infect Immun. 2004 Apr;72(4):2369-78. doi: 10.1128/IAI.72.4.2369-2378.2004. Infect Immun. 2004. PMID: 15039362 Free PMC article.
-
Toll-like receptors and fungal infections: the role of TLR2, TLR4 and MyD88 in paracoccidioidomycosis.FEMS Immunol Med Microbiol. 2008 Jun;53(1):1-7. doi: 10.1111/j.1574-695X.2008.00378.x. Epub 2008 Apr 1. FEMS Immunol Med Microbiol. 2008. PMID: 18384366 Review.
-
Innate immunity to Paracoccidioides brasiliensis infection.Mycopathologia. 2008 Apr-May;165(4-5):223-36. doi: 10.1007/s11046-007-9048-1. Mycopathologia. 2008. PMID: 18777631 Review.
Cited by
-
Regulatory T cells in paracoccidioidomycosis.Virulence. 2019 Dec;10(1):810-821. doi: 10.1080/21505594.2018.1483674. Epub 2018 Aug 1. Virulence. 2019. PMID: 30067137 Free PMC article. Review.
-
ArtinM offers new perspectives in the development of antifungal therapy.Front Microbiol. 2012 Jun 15;3:218. doi: 10.3389/fmicb.2012.00218. eCollection 2012. Front Microbiol. 2012. PMID: 22715337 Free PMC article.
-
Interleukin-1 receptor but not Toll-like receptor 2 is essential for MyD88-dependent Th17 immunity to Coccidioides infection.Infect Immun. 2014 May;82(5):2106-14. doi: 10.1128/IAI.01579-13. Epub 2014 Mar 10. Infect Immun. 2014. PMID: 24614655 Free PMC article.
-
Integrative Analysis of Human Macrophage Inflammatory Response Related to Mycobacterium tuberculosis Virulence.Front Immunol. 2021 Jun 28;12:668060. doi: 10.3389/fimmu.2021.668060. eCollection 2021. Front Immunol. 2021. PMID: 34276658 Free PMC article.
-
Cytokines and the regulation of fungus-specific CD4 T cell differentiation.Cytokine. 2012 Apr;58(1):100-6. doi: 10.1016/j.cyto.2011.11.005. Epub 2011 Nov 30. Cytokine. 2012. PMID: 22133343 Free PMC article. Review.
References
-
- Adachi O., et al. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9:143–150 - PubMed
-
- Biondo C., et al. 2005. MyD88 and TLR2, but not TLR4, are required for host defense against Cryptococcus neoformans. Eur. J. Immunol. 35:870–878 - PubMed
-
- Bonfim C. V., Mamoni R. L., Blotta M. H. 2009. TLR-2, TLR-4 and dectin-1 expression in human monocytes and neutrophils stimulated by Paracoccidioides brasiliensis. Med. Mycol. 47:722–733 - PubMed
-
- Bonifazi P., et al. 2009. Balancing inflammation and tolerance in vivo through dendritic cells by the commensal Candida albicans. Mucosal Immunol. 2:362–374 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

