Dynamics of adherens junctions in epithelial establishment, maintenance, and remodeling
- PMID: 21422226
- PMCID: PMC3063136
- DOI: 10.1083/jcb.201009141
Dynamics of adherens junctions in epithelial establishment, maintenance, and remodeling
Abstract
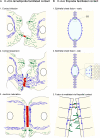
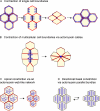
The epithelial cadherin (E-cadherin)-catenin complex binds to cytoskeletal components and regulatory and signaling molecules to form a mature adherens junction (AJ). This dynamic structure physically connects neighboring epithelial cells, couples intercellular adhesive contacts to the cytoskeleton, and helps define each cell's apical-basal axis. Together these activities coordinate the form, polarity, and function of all cells in an epithelium. Several molecules regulate AJ formation and integrity, including Rho family GTPases and Par polarity proteins. However, only recently, with the development of live-cell imaging, has the extent to which E-cadherin is actively turned over at junctions begun to be appreciated. This turnover contributes to junction formation and to the maintenance of epithelial integrity during tissue homeostasis and remodeling.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

