The yeast lipin orthologue Pah1p is important for biogenesis of lipid droplets
- PMID: 21422231
- PMCID: PMC3063132
- DOI: 10.1083/jcb.201010111
The yeast lipin orthologue Pah1p is important for biogenesis of lipid droplets
Abstract
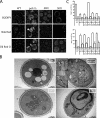
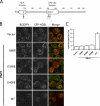
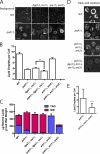
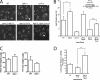
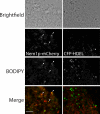
Lipins are phosphatidate phosphatases that generate diacylglycerol (DAG). In this study, we report that yeast lipin, Pah1p, controls the formation of cytosolic lipid droplets. Disruption of PAH1 resulted in a 63% decrease in droplet number, although total neutral lipid levels did not change. This was accompanied by an accumulation of neutral lipids in the endoplasmic reticulum (ER). The droplet biogenesis defect was not a result of alterations in neutral lipid ratios. No droplets were visible in the absence of both PAH1 and steryl acyltransferases when grown in glucose medium, even though the strain produces as much triacylglycerol as wild type. The requirement of PAH1 for normal droplet formation can be bypassed by a knockout of DGK1. Nem1p, the activator of Pah1p, localizes to a single punctum per cell on the ER that is usually next to a droplet, suggesting that it is a site of droplet assembly. Overall, this study provides strong evidence that DAG generated by Pah1p is important for droplet biogenesis.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

