Electrostatic influences of charged inner pore residues on the conductance and gating of small conductance Ca2+ activated K+ channels
- PMID: 21422289
- PMCID: PMC3076840
- DOI: 10.1073/pnas.1103090108
Electrostatic influences of charged inner pore residues on the conductance and gating of small conductance Ca2+ activated K+ channels
Abstract
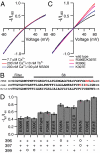
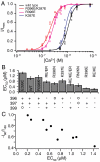
SK channels underlie important physiological functions by linking calcium signaling with neuronal excitability. Potassium currents through SK channels demonstrate inward rectification, which further reduces their small outward conductance. Although it has been generally attributed to block of outward current by intracellular divalent ions, we find that inward rectification is in fact an intrinsic property of SK channels independent of intracellular blockers. We identified three charged residues in the S6 transmembrane domain of SK channels near the inner mouth of the pore that collectively control the conductance and rectification through an electrostatic mechanism. Additionally, electrostatic contributions from these residues also play an important role in determining the intrinsic open probability of SK channels in the absence of Ca(2+), affecting the apparent Ca(2+) affinity for activation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

