Estrogen receptor and progesterone receptor as predictive biomarkers of response to endocrine therapy: a prospectively powered pathology study in the Tamoxifen and Exemestane Adjuvant Multinational trial
- PMID: 21422407
- PMCID: PMC3082973
- DOI: 10.1200/JCO.2010.30.3677
Estrogen receptor and progesterone receptor as predictive biomarkers of response to endocrine therapy: a prospectively powered pathology study in the Tamoxifen and Exemestane Adjuvant Multinational trial
Abstract
Purpose: The Tamoxifen and Exemestane Adjuvant Multinational (TEAM) trial included a prospectively planned pathology substudy testing the predictive value of progesterone receptor (PgR) expression for outcome of estrogen receptor-positive (ER-positive) early breast cancer treated with exemestane versus tamoxifen.
Patients and methods: Pathology blocks from 4,781 TEAM patients randomly assigned to exemestane versus tamoxifen followed by exemestane for 5 years of total therapy were collected centrally, and tissue microarrays were constructed from samples from 4,598 patients. Quantitative analysis of hormone receptors (ER and PgR) was performed by using image analysis and immunohistochemistry, and the results were linked to outcome data from the main TEAM trial and analyzed relative to disease-free survival and treatment.
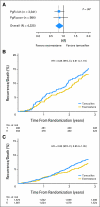
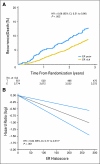
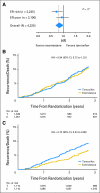
Results: Of 4,325 eligible ER-positive patients, 23% were PgR-poor (Allred < 4) and 77% were PgR- rich (Allred ≥ 5). No treatment-by-marker effect for PgR was observed for exemestane versus tamoxifen (PgR-rich hazard ratio [HR], 0.83; 95% CI, 0.65 to 1.05; PgR-poor HR, 0.85; 95% CI, 0.61 to 1.19; P = .88 for interaction). Both PgR and ER expression were associated with patient prognosis in univariate (PgR HR, 0.53; 95% CI, 0.43 to 0.65; P < .001; ER HR, 0.66; 95% CI, 0.51 to 0.86; P = .002), and multivariate analyses (P < .001 and P = .001, respectively). A trend toward a treatment-by-marker effect for ER-rich patients was observed.
Conclusion: Preferential exemestane versus tamoxifen treatment benefit was not predicted by PgR expression; conversely, patients with ER-rich tumors may derive additional benefit from exemestane. Quantitative analysis of ER and PgR expression provides highly significant information on risk of early relapse (within 1 to 3 years) during treatment.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures


Comment in
-
Can quantifying hormone receptor levels guide the choice of adjuvant endocrine therapy for breast cancer?J Clin Oncol. 2011 Apr 20;29(12):1504-6. doi: 10.1200/JCO.2010.34.3202. Epub 2011 Mar 21. J Clin Oncol. 2011. PMID: 21422423 No abstract available.
References
-
- Tamoxifen for early breast cancer. An overview of the randomised trials—Early Breast Cancer Trialists' Collaborative Group. Lancet. 1998;351:1451–1467. - PubMed
-
- Berstein LM, Wang JP, Zheng H, et al. Long-term exposure to tamoxifen induces hypersensitivity to estradiol. Clin Cancer Res. 2004;10:1530–1534. - PubMed
-
- Fisher B, Dignam J, Bryant J, et al. Five versus more than five years of tamoxifen for lymph node-negative breast cancer: Updated findings from the National Surgical Adjuvant Breast and Bowel Project B-14 randomized trial. J Natl Cancer Inst. 2001;93:684–690. - PubMed
-
- Schiff R, Massarweh S, Shou J, et al. Breast cancer endocrine resistance: How growth factor signaling and estrogen receptor coregulators modulate response. Clin Cancer Res. 2003;9:447S–454S. - PubMed
-
- Witton CJ, Reeves JR, Going JJ, et al. Coexpression of EGFr, HER2, HER3, and HER4 in primary human breast carcinoma. Presented at the 24th Annual San Antonio Breast Cancer Symposium; December 10-13, 2001; San Antonio, TX. abstr 32.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

