Characteristics of human Ewing/PNET sarcoma models
- PMID: 21422656
- PMCID: PMC3102479
- DOI: 10.4103/0256-4947.78206
Characteristics of human Ewing/PNET sarcoma models
Abstract
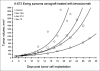
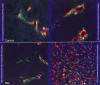
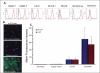
Ewing/PNET (peripheral neuroepithelioma) tumors are rare aggressive bone sarcomas occurring in young people. Rare-disease clinical trials can require global collaborations and many years. In vivo models that as accurately as possible reflect the clinical disease are helpful in selecting therapeutics with the most promise of positive clinical impact. Human Ewing/PNET sarcoma cell lines developed over the past 45 years are described. Several of these have undergone genetic analysis and have been confirmed to be those of Ewing/PNET sarcoma. The A673 Ewing sarcoma line has proven to be particularly useful in understanding the biology of this disease in the mouse. The chromosomal translocation producing the EWS/FLI1 fusion transcript characterizes clinical Ewing sarcoma. Cell lines that express this genetic profile are confirmed to be those of Ewing sarcoma. The A673 Ewing sarcoma line grows in culture and as a xenograft in immunodeficient mice. The A673 model has been used to study Ewing sarcoma angiogenesis and response to antiangiogenic agents. Many Ewing sarcoma clinical specimens express the cell surface protein endosialin. Several Ewing sarcoma cell lines, including the A673 line, also express cell surface endosialin when grown as subcutaneous tumor nodules and as disseminated disease; thus the A673 is a useful model for the study of endosialin biology and endosialin-directed therapies. With the advent of tools that allow characterization of clinical disease to facilitate optimal treatment, it becomes imperative, especially for rare tumors, to develop preclinical models reflecting disease subsets. Ewing PNET sarcomas are a rare disease where models are available.
Figures




References
-
- Bleyer A, Barr R, Hayes-Lattin B, Thomas D, Ellis C, Anderson B. The distinctive biology of cancer in adolescents and young adults. Nature Rev Cancer. 2008;8:288–98. - PubMed
-
- Balamuth NJ, Womer RB. Ewing's sarcoma. Lancet Oncol. 2010;11:184–92. - PubMed
-
- Windsor R, Strauss S, Seddon B, Whelan J. Experimental therapies in Ewing's sarcoma. Expert Opin Investig Drugs. 2009;18:143–59. - PubMed
-
- Chibon F, Lagarde P, Salas S, Perot G, Brouste V, Tirode F, et al. Validated prediction of clinical outcome in sarcomas and multiple types of cancer on the basis of a gene expression signature related to geneome complexity. Nature Med. 2010;16:781–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

