HMGB1 release by inflammasomes
- PMID: 21422809
- PMCID: PMC3265758
- DOI: 10.4161/viru.2.2.15480
HMGB1 release by inflammasomes
Abstract
High-mobility group box 1 (HMGB1) was originally identified as a highly conserved nuclear DNA-binding protein that participates in DNA replication, repair and transcriptional regulation of gene expression. Although the nuclear role of HMGB1 is not quite understood, recent studies characterized the emerging role of extracellular HMGB1 as a prototypical danger signal that regulates inflammatory and repair responses. Under conditions of infection, injury and sterile inflammation, HMGB1 can be passively released from damaged cells or actively secreted from activated immune cells. Inflammasomes, large caspase-1-activating protein complexes, were recently shown to play a critical role in mediating the extracellular release of HMGB1 from activated and infected immune cells.
Figures
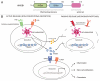
Comment on
-
Inflammasome-dependent release of the alarmin HMGB1 in endotoxemia.J Immunol. 2010 Oct 1;185(7):4385-92. doi: 10.4049/jimmunol.1000803. Epub 2010 Aug 27. J Immunol. 2010. PMID: 20802146 Free PMC article.
References
-
- Goodwin GH, Sanders C, Johns EW. A new group of chromatin-associated proteins with a high content of acidic and basic amino acids. Eur J Biochem. 1973;38:14–19. - PubMed
-
- Hardman CH, Broadhurst RW, Raine AR, Grasser KD, Thomas JO, Laue ED. Structure of the A-domain of HMG1 and its interaction with DNA as studied by heteronuclear three- and four-dimensional NMR spectroscopy. Biochemistry. 1995;34:16596–16607. - PubMed
-
- Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nature Rev Immunol. 2005;5:331–342. - PubMed
-
- Prendergast P, Onate SA, Christensen K, Edwards DP. Nuclear accessory factors enhance the binding of progesterone receptor to specific target DNA. J Steroid Biochem Mol Biol. 1994;48:1–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
