Survey of pharmacologic thromboprophylaxis in critically ill children
- PMID: 21423003
- PMCID: PMC3118917
- DOI: 10.1097/CCM.0b013e3182186ec0
Survey of pharmacologic thromboprophylaxis in critically ill children
Abstract
Objective: There is lack of evidence to guide thromboprophylaxis in the pediatric intensive care unit. We aimed to assess current prescribing practice for pharmacologic thromboprophylaxis in critically ill children.
Setting: Pediatric intensive care units in the United States and Canada with at least ten beds.
Design: Cross-sectional self-administered survey of pediatric intensivists using adolescent, child, and infant scenarios.
Participants: Pediatric intensive care unit clinical directors or section heads.
Interventions: None.
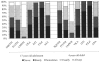
Measurements and main results: Physician leaders from 97 of 151 (64.2%) pediatric intensive care units or their designees responded to the survey. In mechanically ventilated children, 42.3% of the respondents would usually or always prescribe thromboprophylaxis for the adolescent but only 1.0% would prescribe it for the child and 1.1% for the infant. Considering all pediatric intensive care unit patients, 3.1%, 32.0%, and 44.2% of respondents would never prescribe thromboprophylaxis for the adolescent, child, and infant scenarios, respectively. These findings were significant (p < .001 for the adolescent vs. child and infant; p = .002 for child vs. infant). Other patient factors that increased the likelihood of prescribing prophylaxis to a critically ill child for all three scenarios were the presence of hypercoagulability, prior deep venous thrombosis, or a cavopulmonary anastomosis. Prophylaxis was less likely to be prescribed to patients with major bleeding or an anticipated invasive intervention. Low-molecular-weight heparin was the most commonly prescribed drug.
Conclusions: In these scenarios, physician leaders in pediatric intensive care units were more likely to prescribe thromboprophylaxis to adolescents compared with children or infants, but they prescribed it less often in adolescents than is recommended by evidence-based guidelines for adults. The heterogeneity in practice we documented underscores the need for rigorous randomized trials to determine the need for thromboprophylaxis in critically ill adolescents and children.
Conflict of interest statement
The authors have not disclosed any potential conflicts of interest.
Figures

Comment in
-
Thromboprophylaxis in critically ill children: how should we define the "at risk" child?Crit Care Med. 2011 Jul;39(7):1846-7. doi: 10.1097/CCM.0b013e31821cb035. Crit Care Med. 2011. PMID: 21685760 No abstract available.
References
-
- Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133(6 Suppl):381S–453S. - PubMed
-
- Cook D, Crowther M, Meade M, et al. Deep venous thrombosis in medical-surgical critically ill patients: prevalence, incidence, and risk factors. Crit Care Med. 2005;33(7):1565–1571. - PubMed
-
- Raffini L, Huang YS, Witmer C, et al. Dramatic increase in venous thromboembolism in children’s hospitals in the United States from 2001 to 2007. Pediatrics. 2009;124(4):1001–1008. - PubMed
-
- Monagle P, Adams M, Mahoney M, et al. Outcome of pediatric thromboembolic disease: a report from the Canadian Childhood Thrombophilia Registry. Pediatr Res. 2000;47(6):763–766. - PubMed
-
- Monagle P, Chalmers E, Chan A, et al. Antithrombotic therapy in neonates and children: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133(6 Suppl):887S–968S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

