Ecdysteroids affect Drosophila ovarian stem cell niche formation and early germline differentiation
- PMID: 21423150
- PMCID: PMC3102283
- DOI: 10.1038/emboj.2011.73
Ecdysteroids affect Drosophila ovarian stem cell niche formation and early germline differentiation
Abstract
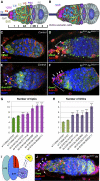
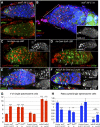
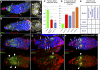
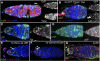
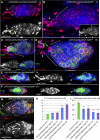
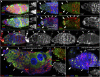
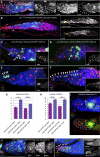
Previously, it has been shown that in Drosophila steroid hormones are required for progression of oogenesis during late stages of egg maturation. Here, we show that ecdysteroids regulate progression through the early steps of germ cell lineage. Upon ecdysone signalling deficit germline stem cell progeny delay to switch on a differentiation programme. This differentiation impediment is associated with reduced TGF-β signalling in the germline and increased levels of cell adhesion complexes and cytoskeletal proteins in somatic escort cells. A co-activator of the ecdysone receptor, Taiman is the spatially restricted regulator of the ecdysone signalling pathway in soma. Additionally, when ecdysone signalling is perturbed during the process of somatic stem cell niche establishment enlarged functional niches able to host additional stem cells are formed.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
Coordinated regulation of niche and stem cell precursors by hormonal signaling.PLoS Biol. 2011 Nov;9(11):e1001202. doi: 10.1371/journal.pbio.1001202. Epub 2011 Nov 22. PLoS Biol. 2011. PMID: 22131903 Free PMC article.
-
Activin signaling balances proliferation and differentiation of ovarian niche precursors and enables adjustment of niche numbers.Development. 2015 Mar 1;142(5):883-92. doi: 10.1242/dev.113902. Epub 2015 Jan 29. Development. 2015. PMID: 25633355
-
Cytoneme-mediated delivery of hedgehog regulates the expression of bone morphogenetic proteins to maintain germline stem cells in Drosophila.PLoS Biol. 2012;10(4):e1001298. doi: 10.1371/journal.pbio.1001298. Epub 2012 Apr 3. PLoS Biol. 2012. PMID: 22509132 Free PMC article.
-
Ecdysone signalling and ovarian development in insects: from stem cells to ovarian follicle formation.Biochim Biophys Acta. 2015 Feb;1849(2):181-6. doi: 10.1016/j.bbagrm.2014.05.025. Epub 2014 Jun 3. Biochim Biophys Acta. 2015. PMID: 24939835 Review.
-
Molecular control of the female germline stem cell niche size in Drosophila.Cell Mol Life Sci. 2019 Nov;76(21):4309-4317. doi: 10.1007/s00018-019-03223-0. Epub 2019 Jul 12. Cell Mol Life Sci. 2019. PMID: 31300869 Free PMC article. Review.
Cited by
-
Steroids as external temporal codes act via microRNAs and cooperate with cytokines in differential neurogenesis.Fly (Austin). 2013 Jul-Sep;7(3):173-83. doi: 10.4161/fly.25241. Epub 2013 Jul 9. Fly (Austin). 2013. PMID: 23839338 Free PMC article.
-
Distinct gene expression dynamics in germ line and somatic tissue during ovariole morphogenesis in Drosophila melanogaster.G3 (Bethesda). 2022 Feb 4;12(2):jkab305. doi: 10.1093/g3journal/jkab305. G3 (Bethesda). 2022. PMID: 34849771 Free PMC article.
-
Genetic basis for developmental homeostasis of germline stem cell niche number: a network of Tramtrack-Group nuclear BTB factors.PLoS One. 2012;7(11):e49958. doi: 10.1371/journal.pone.0049958. Epub 2012 Nov 21. PLoS One. 2012. PMID: 23185495 Free PMC article.
-
Niche formation and function in developing tissue: studies from the Drosophila ovary.Cell Commun Signal. 2023 Jan 27;21(1):23. doi: 10.1186/s12964-022-01035-7. Cell Commun Signal. 2023. PMID: 36707894 Free PMC article. Review.
-
Ecdysone response gene E78 controls ovarian germline stem cell niche formation and follicle survival in Drosophila.Dev Biol. 2015 Apr 1;400(1):33-42. doi: 10.1016/j.ydbio.2015.01.013. Epub 2015 Jan 23. Dev Biol. 2015. PMID: 25624267 Free PMC article.
References
-
- Bai J, Uehara Y, Montell DJ (2000) Regulation of invasive cell behavior by taiman, a Drosophila protein related to AIB1, a steroid receptor coactivator amplified in breast cancer. Cell 103: 1047–1058 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

