Crystal structure of inhibitor of κB kinase β
- PMID: 21423167
- PMCID: PMC3081413
- DOI: 10.1038/nature09853
Crystal structure of inhibitor of κB kinase β
Abstract
Inhibitor of κB (IκB) kinase (IKK) phosphorylates IκB proteins, leading to their degradation and the liberation of nuclear factor κB for gene transcription. Here we report the crystal structure of IKKβ in complex with an inhibitor, at a resolution of 3.6 Å. The structure reveals a trimodular architecture comprising the kinase domain, a ubiquitin-like domain (ULD) and an elongated, α-helical scaffold/dimerization domain (SDD). Unexpectedly, the predicted leucine zipper and helix-loop-helix motifs do not form these structures but are part of the SDD. The ULD and SDD mediate a critical interaction with IκBα that restricts substrate specificity, and the ULD is also required for catalytic activity. The SDD mediates IKKβ dimerization, but dimerization per se is not important for maintaining IKKβ activity and instead is required for IKKβ activation. Other IKK family members, IKKα, TBK1 and IKK-i, may have a similar trimodular architecture and function.
Figures
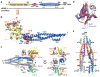
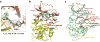



Comment in
-
Protein structure: 'shear' clarity of IKKβ crystal.Nat Rev Drug Discov. 2011 Apr;10(4):260. doi: 10.1038/nrd3424. Nat Rev Drug Discov. 2011. PMID: 21455235 No abstract available.
References
-
- Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132 (3):344. - PubMed
-
- Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693. - PubMed
-
- Scheidereit C. IkappaB kinase complexes: gateways to NF-kappaB activation and transcription. Oncogene. 2006;25 (51):6685. - PubMed
-
- Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441 (7092):431. - PubMed
-
- Chen ZJ, Parent L, Maniatis T. Site-specific phosphorylation of IkappaBalpha by a novel ubiquitination-dependent protein kinase activity. Cell. 1996;84 (6):853. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

