Analysis of the myosin-II-responsive focal adhesion proteome reveals a role for β-Pix in negative regulation of focal adhesion maturation
- PMID: 21423176
- PMCID: PMC3279191
- DOI: 10.1038/ncb2216
Analysis of the myosin-II-responsive focal adhesion proteome reveals a role for β-Pix in negative regulation of focal adhesion maturation
Abstract
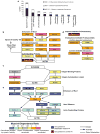
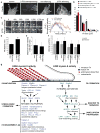
Focal adhesions undergo myosin-II-mediated maturation wherein they grow and change composition to modulate integrin signalling for cell migration, growth and differentiation. To determine how focal adhesion composition is affected by myosin II activity, we performed proteomic analysis of isolated focal adhesions and compared protein abundance in focal adhesions from cells with and without myosin II inhibition. We identified 905 focal adhesion proteins, 459 of which changed in abundance with myosin II inhibition, defining the myosin-II-responsive focal adhesion proteome. The abundance of 73% of the proteins in the myosin-II-responsive focal adhesion proteome was enhanced by contractility, including proteins involved in Rho-mediated focal adhesion maturation and endocytosis- and calpain-dependent focal adhesion disassembly. During myosin II inhibition, 27% of proteins in the myosin-II-responsive focal adhesion proteome, including proteins involved in Rac-mediated lamellipodial protrusion, were enriched in focal adhesions, establishing that focal adhesion protein recruitment is also negatively regulated by contractility. We focused on the Rac guanine nucleotide exchange factor β-Pix, documenting its role in the negative regulation of focal adhesion maturation and the promotion of lamellipodial protrusion and focal adhesion turnover to drive cell migration.
© 2011 Macmillan Publishers Limited. All rights reserved
Figures





Comment in
-
The myosin-II-responsive focal adhesion proteome: a tour de force?Nat Cell Biol. 2011 Apr;13(4):344-6. doi: 10.1038/ncb0411-344. Epub 2011 Mar 20. Nat Cell Biol. 2011. PMID: 21460805
-
Cell migration: keeping young and mobile with β-PIX.Nat Rev Mol Cell Biol. 2011 May;12(5):278. doi: 10.1038/nrm3104. Epub 2011 Apr 7. Nat Rev Mol Cell Biol. 2011. PMID: 21472000 No abstract available.
References
-
- Burridge K, Fath K, Kelly T, Nuckolls G, Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. - PubMed
-
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. - PubMed
-
- Bershadsky A, Kozlov M, Geiger B. Adhesion-mediated mechanosensitivity: a time to experiment, and a time to theorize. Curr Opin Cell Biol. 2006;18:472–481. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

