Homology-directed Fanconi anemia pathway cross-link repair is dependent on DNA replication
- PMID: 21423196
- PMCID: PMC3273992
- DOI: 10.1038/nsmb.2029
Homology-directed Fanconi anemia pathway cross-link repair is dependent on DNA replication
Abstract
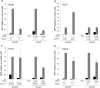
Homologous recombination (also termed homology-directed repair, HDR) is a major pathway for the repair of DNA interstrand cross-links (ICLs) in mammalian cells. Cells from individuals with Fanconi anemia (FA) are characterized by extreme ICL sensitivity, but their reported defect in HDR is mild. Here we examined ICL-induced HDR using a GFP reporter and observed a profound defect in ICL-induced HDR in FA cells, but only when the reporter could replicate.
Figures

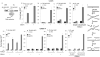
References
-
- Wang W. Nat Rev Genet. 2007;8:735–748. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

