HGF-independent potentiation of EGFR action by c-Met
- PMID: 21423210
- PMCID: PMC3126872
- DOI: 10.1038/onc.2011.84
HGF-independent potentiation of EGFR action by c-Met
Abstract
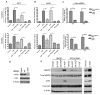
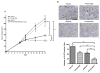
The c-Met receptor is a potential therapeutic target for non-small cell lung cancer (NSCLC). Signaling interactions between c-Met and the mutant epidermal growth factor receptor (EGFR) have been studied extensively, but signaling intermediates and biological consequences of lateral signaling to c-Met in EGFR wild-type tumors are minimally understood. Our observations indicate that delayed c-Met activation in NSCLC cell lines is initiated by wild-type EGFR, the receptor most often found in NSCLC tumors. EGFR ligands induce accumulation of activated c-Met, which begins at 8 h and continues for 48 h. This effect is accompanied by an increase in c-Met expression and phosphorylation of critical c-Met tyrosine residues without activation of mitogen-activated protein kinase (MAPK) or Akt. Gene transcription is required for delayed c-Met activation; however, phosphorylation of c-Met by EGFR occurs without production of hepatocyte growth factor (HGF) or another secreted factor, supporting a ligand-independent mechanism. Lateral signaling is blocked by two selective c-Met tyrosine kinase inhibitors (TKIs), PF2341066 and SU11274, or with gefitinib, an EGFR TKI, suggesting kinase activity of both receptors is required for this effect. Prolonged c-Src phosphorylation is observed, and c-Src pathway is essential for EGFR to c-Met communication. Pretreatment with pan-Src family kinase inhibitors, PP2 and dasatinib, abolishes delayed c-Met phosphorylation. A c-Src dominant-negative construct reduces EGF-induced c-Met phosphorylation compared with control, further confirming a c-Src requirement. Inhibition of c-Met with PF2341066 and siRNA decreases EGF-induced phenotypes of invasion by ~86% and motility by ~81%, suggesting that a novel form of c-Met activation is utilized by EGFR to maximize these biological effects. Combined targeting of c-Met and EGFR leads to increased xenograft antitumor activity, demonstrating that inhibition of downstream and lateral signaling from the EGFR-c-Src-c-Met axis might be effective in treatment of NSCLC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Bergstrom JD, Westermark B, Heldin NE. Exp Cell Res. 2000;259:293–9. - PubMed
-
- Bottaro DP, Rubin JS, Faletto DL, Chan AM, Kmiecik TE, Vande Woude GF, Aaronson SA. Science. 1991;251:802–4. - PubMed
-
- Danilkovitch-Miagkova A, Angeloni D, Skeel A, Donley S, Lerman M, Leonard EJ. J Biol Chem. 2000;275:14783–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

