Rapid death of duck cells infected with influenza: a potential mechanism for host resistance to H5N1
- PMID: 21423263
- PMCID: PMC3257048
- DOI: 10.1038/icb.2011.17
Rapid death of duck cells infected with influenza: a potential mechanism for host resistance to H5N1
Abstract
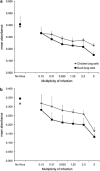
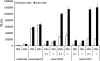
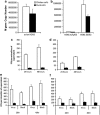
Aquatic birds are the natural reservoir for most subtypes of influenza A, and a source of novel viruses with the potential to cause human pandemics, fatal zoonotic disease or devastating epizootics in poultry. It is well recognised that waterfowl typically show few clinical signs following influenza A infection, in contrast, terrestrial poultry such as chickens may develop severe disease with rapid death following infection with highly pathogenic avian influenza. This study examined the cellular response to influenza infection in primary cells derived from resistant (duck) and susceptible (chicken) avian hosts. Paradoxically, we observed that duck cells underwent rapid cell death following infection with low pathogenic avian H2N3, classical swine H1N1 and 'classical' highly pathogenic H5N1 viruses. Dying cells showed morphological features of apoptosis, increased DNA fragmentation and activation of caspase 3/7. Following infection of chicken cells, cell death occurred less rapidly, accompanied by reduced DNA fragmentation and caspase activation. Duck cells produced similar levels of viral RNA but less infectious virus, in comparison with chicken cells. Such rapid cell death was not observed in duck cells infected with a contemporary Eurasian lineage H5N1 fatal to ducks. The induction of rapid death in duck cells may be part of a mechanism of host resistance to influenza A, with the loss of this response leading to increased susceptibility to emergent strains of H5N1. These studies provide novel insights that should help resolve the long-standing enigma of host-pathogen relationships for highly pathogenic and zoonotic avian influenza.
Figures


Similar articles
-
Chicken and duck myotubes are highly susceptible and permissive to influenza virus infection.J Virol. 2015 Mar;89(5):2494-506. doi: 10.1128/JVI.03421-14. Epub 2014 Dec 24. J Virol. 2015. PMID: 25540384 Free PMC article.
-
Highly pathogenic avian influenza virus infection in chickens but not ducks is associated with elevated host immune and pro-inflammatory responses.Vet Res. 2014 Nov 28;45(1):118. doi: 10.1186/s13567-014-0118-3. Vet Res. 2014. PMID: 25431115 Free PMC article.
-
Identification of morphological differences between avian influenza A viruses grown in chicken and duck cells.Virus Res. 2015 Mar 2;199:9-19. doi: 10.1016/j.virusres.2015.01.005. Epub 2015 Jan 19. Virus Res. 2015. PMID: 25613009
-
Ducks: the "Trojan horses" of H5N1 influenza.Influenza Other Respir Viruses. 2009 Jul;3(4):121-8. doi: 10.1111/j.1750-2659.2009.00084.x. Influenza Other Respir Viruses. 2009. PMID: 19627369 Free PMC article. Review.
-
Moving H5N1 studies into the era of systems biology.Virus Res. 2013 Dec 5;178(1):151-67. doi: 10.1016/j.virusres.2013.02.011. Epub 2013 Mar 14. Virus Res. 2013. PMID: 23499671 Free PMC article. Review.
Cited by
-
The quail genome: insights into social behaviour, seasonal biology and infectious disease response.BMC Biol. 2020 Feb 12;18(1):14. doi: 10.1186/s12915-020-0743-4. BMC Biol. 2020. PMID: 32050986 Free PMC article.
-
Insight into alternative approaches for control of avian influenza in poultry, with emphasis on highly pathogenic H5N1.Viruses. 2012 Nov 19;4(11):3179-208. doi: 10.3390/v4113179. Viruses. 2012. PMID: 23202521 Free PMC article. Review.
-
Molecular basis of the structure and function of H1 hemagglutinin of influenza virus.Proc Jpn Acad Ser B Phys Biol Sci. 2012;88(6):226-49. doi: 10.2183/pjab.88.226. Proc Jpn Acad Ser B Phys Biol Sci. 2012. PMID: 22728439 Free PMC article. Review.
-
Toll-like receptor responses to Peste des petits ruminants virus in goats and water buffalo.PLoS One. 2014 Nov 4;9(11):e111609. doi: 10.1371/journal.pone.0111609. eCollection 2014. PLoS One. 2014. PMID: 25369126 Free PMC article.
-
Pathogenicity and tissue tropism of infectious bronchitis virus is associated with elevated apoptosis and innate immune responses.Virology. 2016 Jan 15;488:232-41. doi: 10.1016/j.virol.2015.11.011. Epub 2015 Dec 3. Virology. 2016. PMID: 26655241 Free PMC article.
References
-
- Horimoto T, Kawaoka Y. Influenza: lessons from past pandemics, warnings from current incidents. Nat Rev Microbiol. 2005;3:591–600. - PubMed
-
- Li KS, Guan Y, Wang J, Smith GJ, Xu KM, Duan L, et al. Genesis of a highly pathogenic and potentially pandemic H5N1 influenza virus in eastern Asia. Nature. 2004;430:209–213. - PubMed
-
- World Health Organisation Cumulative Number of Confirmed Human Cases of Avian Influenza A/(H5N1) Reported to WHO2010
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

