PTBP1 is required for embryonic development before gastrulation
- PMID: 21423341
- PMCID: PMC3040740
- DOI: 10.1371/journal.pone.0016992
PTBP1 is required for embryonic development before gastrulation
Abstract
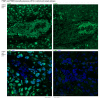
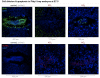
Polypyrimidine-tract binding protein 1 (PTBP1) is an important cellular regulator of messenger RNAs influencing the alternative splicing profile of a cell as well as its mRNA stability, location and translation. In addition, it is diverted by some viruses to facilitate their replication. Here, we used a novel PTBP1 knockout mouse to analyse the tissue expression pattern of PTBP1 as well as the effect of its complete removal during development. We found evidence of strong PTBP1 expression in embryonic stem cells and throughout embryonic development, especially in the developing brain and spinal cord, the olfactory and auditory systems, the heart, the liver, the kidney, the brown fat and cartilage primordia. This widespread distribution points towards a role of PTBP1 during embryonic development. Homozygous offspring, identified by PCR and immunofluorescence, were able to implant but were arrested or retarded in growth. At day 7.5 of embryonic development (E7.5) the null mutants were about 5x smaller than the control littermates and the gap in body size widened with time. At mid-gestation, all homozygous embryos were resorbed/degraded. No homozygous mice were genotyped at E12 and the age of weaning. Embryos lacking PTBP1 did not display differentiation into the 3 germ layers and cavitation of the epiblast, which are hallmarks of gastrulation. In addition, homozygous mutants displayed malformed ectoplacental cones and yolk sacs, both early supportive structure of the embryo proper. We conclude that PTBP1 is not required for the earliest isovolumetric divisions and differentiation steps of the zygote up to the formation of the blastocyst. However, further post-implantation development requires PTBP1 and stalls in homozygous null animals with a phenotype of dramatically reduced size and aberration in embryonic and extra-embryonic structures.
Conflict of interest statement
Figures






Similar articles
-
Comparative analysis of human and mouse development: From zygote to pre-gastrulation.Curr Top Dev Biol. 2020;136:113-138. doi: 10.1016/bs.ctdb.2019.10.002. Epub 2019 Dec 26. Curr Top Dev Biol. 2020. PMID: 31959285 Review.
-
Early human embryonic development: Blastocyst formation to gastrulation.Dev Cell. 2022 Jan 24;57(2):152-165. doi: 10.1016/j.devcel.2021.12.022. Dev Cell. 2022. PMID: 35077679 Review.
-
PTBP1 contributes to spermatogenesis through regulation of proliferation in spermatogonia.J Reprod Dev. 2019 Feb 8;65(1):37-46. doi: 10.1262/jrd.2018-109. Epub 2018 Nov 12. J Reprod Dev. 2019. PMID: 30416150 Free PMC article.
-
Nhlrc2 is crucial during mouse gastrulation.Genesis. 2022 Mar;60(3):e23470. doi: 10.1002/dvg.23470. Epub 2022 Mar 8. Genesis. 2022. PMID: 35258166 Free PMC article.
-
Embryonic Cul4b is important for epiblast growth and location of primitive streak layer cells.PLoS One. 2019 Jul 1;14(7):e0219221. doi: 10.1371/journal.pone.0219221. eCollection 2019. PLoS One. 2019. PMID: 31260508 Free PMC article.
Cited by
-
EMT and stemness: flexible processes tuned by alternative splicing in development and cancer progression.Mol Cancer. 2017 Jan 30;16(1):8. doi: 10.1186/s12943-016-0579-2. Mol Cancer. 2017. PMID: 28137272 Free PMC article. Review.
-
Uncovering RNA binding proteins associated with age and gender during liver maturation.Sci Rep. 2015 Mar 31;5:9512. doi: 10.1038/srep09512. Sci Rep. 2015. PMID: 25824884 Free PMC article.
-
RNA-binding protein Ptbp1 regulates alternative splicing and transcriptome in spermatogonia and maintains spermatogenesis in concert with Nanos3.J Reprod Dev. 2020 Oct 13;66(5):459-467. doi: 10.1262/jrd.2020-060. Epub 2020 Jul 6. J Reprod Dev. 2020. PMID: 32624547 Free PMC article.
-
Specific inhibition of splicing factor activity by decoy RNA oligonucleotides.Nat Commun. 2019 Apr 8;10(1):1590. doi: 10.1038/s41467-019-09523-0. Nat Commun. 2019. PMID: 30962446 Free PMC article.
-
Neuronal regulation of pre-mRNA splicing by polypyrimidine tract binding proteins, PTBP1 and PTBP2.Crit Rev Biochem Mol Biol. 2012 Jul-Aug;47(4):360-78. doi: 10.3109/10409238.2012.691456. Epub 2012 Jun 2. Crit Rev Biochem Mol Biol. 2012. PMID: 22655688 Free PMC article.
References
-
- Wang H, Dey SK. Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet. 2006;7:185–199. - PubMed
-
- Tam PPL, Loebel DAF. Gene function in mouse embryogenesis: get set for gastrulation. Nat Rev Genet. 2007;8:368–381. - PubMed
-
- Cross JC. How to make a placenta: mechanisms of trophoblast cell differentiation in mice–a review. Placenta. 2005;26(Suppl A):S3–9. - PubMed
-
- Wollerton MC, Gooding C, Wagner EJ, Garcia-Blanco MA, Smith CWJ. Autoregulation of polypyrimidine tract binding protein by alternative splicing leading to nonsense-mediated decay. Mol Cell. 2004;13:91–100. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

