Alternans in genetically modified langendorff-perfused murine hearts modeling catecholaminergic polymorphic ventricular tachycardia
- PMID: 21423368
- PMCID: PMC3059940
- DOI: 10.3389/fphys.2010.00126
Alternans in genetically modified langendorff-perfused murine hearts modeling catecholaminergic polymorphic ventricular tachycardia
Abstract
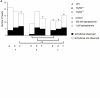
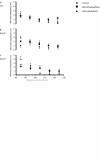
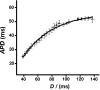
The relationship between alternans and arrhythmogenicity was studied in genetically modified murine hearts modeling catecholaminergic polymorphic ventricular tachycardia (CPVT) during Langendorff perfusion, before and after treatment with catecholamines and a β-adrenergic antagonist. Heterozygous (RyR2(p/s)) and homozygous (RyR2(s/s)) RyR2-P2328S hearts, and wild-type (WT) controls, were studied before and after treatment with epinephrine (100 nM and 1 μM) and propranolol (100 nM). Monophasic action potential recordings demonstrated significantly greater incidences of arrhythmia in RyR2(p/s) and RyR2(s/s) hearts as compared to WTs. Arrhythmogenicity in RyR2(s/s) hearts was associated with alternans, particularly at short baseline cycle lengths. Both phenomena were significantly accentuated by treatment with epinephrine and significantly diminished by treatment with propranolol, in full agreement with clinical expectations. These changes took place, however, despite an absence of changes in mean action potential durations, ventricular effective refractory periods or restitution curve characteristics. Furthermore pooled data from all hearts in which arrhythmia occurred demonstrated significantly greater alternans magnitudes, but similar restitution curve slopes, to hearts that did not demonstrate arrhythmia. These findings thus further validate the RyR2-P2328S murine heart as a model for human CPVT, confirming an alternans phenotype in common with murine genetic models of the Brugada syndrome and the congenital long-QT syndrome type 3. In contrast to these latter similarities, however, this report demonstrates the dissociation of alternans from changes in the properties of restitution curves for the first time in a murine model of a human arrhythmic syndrome.
Keywords: Brugada syndrome; alternans; arrhythmia; catecholaminergic polymorphic ventricular tachycardia; monophasic action potential; restitution curve; ryanodine receptor; sudden cardiac death.
Figures


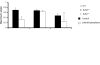
Similar articles
-
The RyR2-P2328S mutation downregulates Nav1.5 producing arrhythmic substrate in murine ventricles.Pflugers Arch. 2016 Apr;468(4):655-65. doi: 10.1007/s00424-015-1750-0. Epub 2015 Nov 6. Pflugers Arch. 2016. PMID: 26545784 Free PMC article.
-
Flecainide exerts paradoxical effects on sodium currents and atrial arrhythmia in murine RyR2-P2328S hearts.Acta Physiol (Oxf). 2015 Jul;214(3):361-75. doi: 10.1111/apha.12505. Epub 2015 Apr 23. Acta Physiol (Oxf). 2015. PMID: 25850710 Free PMC article.
-
Nonlinearity between action potential alternans and restitution, which both predict ventricular arrhythmic properties in Scn5a+/- and wild-type murine hearts.J Appl Physiol (1985). 2012 Jun;112(11):1847-63. doi: 10.1152/japplphysiol.00039.2012. Epub 2012 Mar 29. J Appl Physiol (1985). 2012. PMID: 22461438 Free PMC article.
-
Catecholaminergic polymorphic ventricular tachycardia: recent mechanistic insights.Cardiovasc Res. 2005 Aug 15;67(3):379-87. doi: 10.1016/j.cardiores.2005.04.027. Cardiovasc Res. 2005. PMID: 15913575 Review.
-
[Genetic of catecholaminergic polymorphic ventricular tachycardia: basic concepts].Arch Cardiol Mex. 2009 Dec;79 Suppl 2:13-7. Arch Cardiol Mex. 2009. PMID: 20361477 Review. Spanish.
Cited by
-
Cardiac electrophysiological adaptations in the equine athlete-Restitution analysis of electrocardiographic features.PLoS One. 2018 Mar 9;13(3):e0194008. doi: 10.1371/journal.pone.0194008. eCollection 2018. PLoS One. 2018. PMID: 29522557 Free PMC article.
-
Calcium alternans in a couplon network model of ventricular myocytes: role of sarcoplasmic reticulum load.Am J Physiol Heart Circ Physiol. 2012 Aug 1;303(3):H341-52. doi: 10.1152/ajpheart.00302.2012. Epub 2012 Jun 1. Am J Physiol Heart Circ Physiol. 2012. PMID: 22661509 Free PMC article.
-
Formation of spatially discordant alternans due to fluctuations and diffusion of calcium.PLoS One. 2013 Dec 31;8(12):e85365. doi: 10.1371/journal.pone.0085365. eCollection 2013. PLoS One. 2013. PMID: 24392005 Free PMC article.
-
Ion channel gating in cardiac ryanodine receptors from the arrhythmic RyR2-P2328S mouse.J Cell Sci. 2019 May 21;132(10):jcs229039. doi: 10.1242/jcs.229039. J Cell Sci. 2019. PMID: 31028179 Free PMC article.
-
Arrhythmogenic mechanism of a novel ryanodine receptor mutation underlying sudden cardiac death.Europace. 2023 Jul 4;25(7):euad220. doi: 10.1093/europace/euad220. Europace. 2023. PMID: 37466361 Free PMC article.
References
-
- Antzelevitch C., Burashnikov A., Di Diego J. M. (2003). “Cellular and ionic mechanisms underlying arrhythmogenesis,” in Cardiac Repolarization: Bridging Basic and Clinical Science, eds Gussak I., Antzelevitch C., Hammill S. C., Shen W. K. (Totowa, NJ: Humana Press; ), 201–252
-
- Cerrone M., Colombi B., Santoro M., di Barletta M. R., Scelsi M., Villani L., Napolitano C., Priori S. G. (2005). Bidirectional ventricular tachycardia and fibrillation elicited in a knock-in mouse model carrier of a mutation in the cardiac ryanodine receptor. Circ. Res. 96, e77–e8210.1161/01.RES.0000169067.51055.72 - DOI - PubMed
-
- Fabritz L., Kirchhof P., Franz M. R., Eckardt L., Monnig G., Milberg P., Breithardt G., Haverkamp W. (2003). Prolonged action potential durations, increased dispersion of repolarization, and polymorphic ventricular tachycardia in a mouse model of proarrhythmia. Basic Res. Cardiol. 98, 25–3210.1007/s00395-003-0386-y - DOI - PubMed
LinkOut - more resources
Full Text Sources

