Effect of PPARγ inhibition during pregnancy on posterior cerebral artery function and structure
- PMID: 21423372
- PMCID: PMC3059960
- DOI: 10.3389/fphys.2010.00130
Effect of PPARγ inhibition during pregnancy on posterior cerebral artery function and structure
Abstract
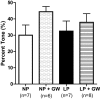
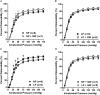
Peroxisome proliferator-activated receptor-γ (PPARγ), a ligand-activated transcription factor, has protective roles in the cerebral circulation and is highly activated during pregnancy. Thus, we hypothesized that PPARγ is involved in the adaptation of cerebral vasculature to pregnancy. Non-pregnant (NP) and late-pregnant (LP) rats were treated with a specific PPARγ inhibitor GW9662 (10 ]mg/kg/day, in food) or vehicle for 10 days and vascular function and structural remodeling were determined in isolated and pressurized posterior cerebral arteries (PCA). Expression of PPARγ and angiotensin type 1 receptor (AT1R) in cerebral (pial) vessels was determined by real-time RT-PCR. PPARγ inhibition decreased blood pressure and increased blood glucose in NP rats, but not in LP rats. PPARγ inhibition reduced dilation to acetylcholine and sodium nitroprusside in PCA from NP (p < 0.05 vs. LP-GW), but not LP rats. PPARγ inhibition tended to increase basal tone and myogenic activity in PCA from NP rats, but not LP rats. Structurally, PPARγ inhibition increased wall thickness in PCA from both NP and LP rats (p < 0.05), but increased distensibility only in PCA from NP rats. Pregnancy decreased expression of PPARγ and AT1R (p < 0.05) in cerebral arteries that was not affected by GW9662 treatment. These results suggest that PPARγ inhibition had significant effects on the function and structure of PCA in the NP state, but appeared to have less influence during pregnancy. Down-regulation of PPARγ and AT1R in cerebral arteries may be responsible for the lack of effect of PPARγ in cerebral vasculature and may be part of the vascular adaptation to pregnancy.
Keywords: peroxisome proliferator-activated receptor-γ; posterior cerebral arteries; pregnancy; vascular remodeling; vasodilation.
Figures
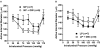
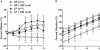
Similar articles
-
Relaxin causes selective outward remodeling of brain parenchymal arterioles via activation of peroxisome proliferator-activated receptor-γ.FASEB J. 2011 Sep;25(9):3229-39. doi: 10.1096/fj.10-175471. Epub 2011 May 20. FASEB J. 2011. PMID: 21602449 Free PMC article.
-
Cerebral artery reactivity changes during pregnancy and the postpartum period: a role in eclampsia?Am J Physiol Heart Circ Physiol. 2004 Jun;286(6):H2127-32. doi: 10.1152/ajpheart.01154.2003. Epub 2004 Jan 29. Am J Physiol Heart Circ Physiol. 2004. PMID: 14751854
-
Inhibition of PPARγ during rat pregnancy causes intrauterine growth restriction and attenuation of uterine vasodilation.Front Physiol. 2013 Jul 23;4:184. doi: 10.3389/fphys.2013.00184. eCollection 2013. Front Physiol. 2013. PMID: 23888144 Free PMC article.
-
Pregnancy prevents hypertensive remodeling of cerebral arteries: a potential role in the development of eclampsia.Hypertension. 2006 Mar;47(3):619-26. doi: 10.1161/01.HYP.0000196948.15019.28. Epub 2005 Dec 27. Hypertension. 2006. PMID: 16380541
-
Impact of peroxisome proliferator-activated receptor γ on angiotensin II type 1 receptor-mediated insulin sensitivity, vascular inflammation and atherogenesis in hypercholesterolemic mice.Arch Med Sci. 2015 Aug 12;11(4):877-85. doi: 10.5114/aoms.2015.53309. Epub 2015 Aug 11. Arch Med Sci. 2015. PMID: 26322101 Free PMC article.
Cited by
-
Improvement in middle cerebral artery structure and endothelial function in stroke-prone spontaneously hypertensive rats after macrophage depletion.Microcirculation. 2013 Oct;20(7):650-61. doi: 10.1111/micc.12064. Microcirculation. 2013. PMID: 23647512 Free PMC article.
-
Rigid and remodelled: cerebrovascular structure and function after experimental high-thoracic spinal cord transection.J Physiol. 2016 Mar 15;594(6):1677-88. doi: 10.1113/JP270925. Epub 2016 Jan 18. J Physiol. 2016. PMID: 26634420 Free PMC article.
-
Relaxin causes selective outward remodeling of brain parenchymal arterioles via activation of peroxisome proliferator-activated receptor-γ.FASEB J. 2011 Sep;25(9):3229-39. doi: 10.1096/fj.10-175471. Epub 2011 May 20. FASEB J. 2011. PMID: 21602449 Free PMC article.
-
Altered hippocampal arteriole structure and function in a rat model of preeclampsia: Potential role in impaired seizure-induced hyperemia.J Cereb Blood Flow Metab. 2017 Aug;37(8):2857-2869. doi: 10.1177/0271678X16676287. Epub 2016 Jan 1. J Cereb Blood Flow Metab. 2017. PMID: 27815419 Free PMC article.
-
Candesartan prevents arteriopathy progression in cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy model.J Clin Invest. 2021 Nov 15;131(22):e140555. doi: 10.1172/JCI140555. J Clin Invest. 2021. PMID: 34779414 Free PMC article.
References
-
- Baumbach G. L., Dobrin P. B., Hart M. N., Heistad D. D. (1988). Mechanics of cerebral arterioles in hypertensive rats. Circ. Res. 62, 56–64 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

