Physiology and pathophysiology of ClC-K/barttin channels
- PMID: 21423394
- PMCID: PMC3059957
- DOI: 10.3389/fphys.2010.00155
Physiology and pathophysiology of ClC-K/barttin channels
Abstract
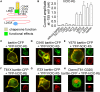
ClC-K channels form a subgroup of anion channels within the ClC family of anion transport proteins. They are expressed predominantly in the kidney and in the inner ear, and are necessary for NaCl resorption in the loop of Henle and for K+ secretion by the stria vascularis. Subcellular distribution as well as the function of these channels are tightly regulated by an accessory subunit, barttin. Barttin improves the stability of ClC-K channel protein, stimulates the exit from the endoplasmic reticulum and insertion into the plasma membrane and changes its function by modifying voltage-dependent gating processes. The importance of ClC-K/barttin channels is highlighted by several genetic diseases. Dysfunctions of ClC-K channels result in Bartter syndrome, an inherited human condition characterized by impaired urinary concentration. Mutations in the gene encoding barttin, BSND, affect the urinary concentration as well as the sensory function of the inner ear. Surprisingly, there is one BSND mutation that causes deafness without affecting renal function, indicating that kidney function tolerates a reduction of anion channel activity that is not sufficient to support normal signal transduction in inner hair cells. This review summarizes recent work on molecular mechanisms, physiology, and pathophysiology of ClC-K/barttin channels.
Keywords: ClC channels; barttin; epithelial transport; loop of Henle; stria vascularis.
Figures
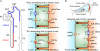



Similar articles
-
Reduced Membrane Insertion of CLC-K by V33L Barttin Results in Loss of Hearing, but Leaves Kidney Function Intact.Front Physiol. 2017 May 15;8:269. doi: 10.3389/fphys.2017.00269. eCollection 2017. Front Physiol. 2017. PMID: 28555110 Free PMC article.
-
Activation of renal ClC-K chloride channels depends on an intact N terminus of their accessory subunit barttin.J Biol Chem. 2018 Jun 1;293(22):8626-8637. doi: 10.1074/jbc.RA117.000860. Epub 2018 Apr 19. J Biol Chem. 2018. PMID: 29674316 Free PMC article.
-
Human CLC-K Channels Require Palmitoylation of Their Accessory Subunit Barttin to Be Functional.J Biol Chem. 2015 Jul 10;290(28):17390-400. doi: 10.1074/jbc.M114.631705. Epub 2015 May 26. J Biol Chem. 2015. PMID: 26013830 Free PMC article.
-
Mechanisms of Disease: the kidney-specific chloride channels ClCKA and ClCKB, the Barttin subunit, and their clinical relevance.Nat Clin Pract Nephrol. 2008 Jan;4(1):38-46. doi: 10.1038/ncpneph0689. Nat Clin Pract Nephrol. 2008. PMID: 18094726 Review.
-
Small Molecules Targeting Kidney ClC-K Chloride Channels: Applications in Rare Tubulopathies and Common Cardiovascular Diseases.Biomolecules. 2023 Apr 21;13(4):710. doi: 10.3390/biom13040710. Biomolecules. 2023. PMID: 37189456 Free PMC article. Review.
Cited by
-
Bartter syndrome with long-term follow-up: a case report.J Int Med Res. 2020 Aug;48(8):300060520947876. doi: 10.1177/0300060520947876. J Int Med Res. 2020. PMID: 32857947 Free PMC article.
-
New Insights into the Mechanism of NO3- Selectivity in the Human Kidney Chloride Channel ClC-Ka and the CLC Protein Family.J Am Soc Nephrol. 2019 Feb;30(2):293-302. doi: 10.1681/ASN.2018060593. Epub 2019 Jan 11. J Am Soc Nephrol. 2019. PMID: 30635372 Free PMC article.
-
Functional Study of Novel Bartter's Syndrome Mutations in ClC-Kb and Rescue by the Accessory Subunit Barttin Toward Personalized Medicine.Front Pharmacol. 2020 Mar 17;11:327. doi: 10.3389/fphar.2020.00327. eCollection 2020. Front Pharmacol. 2020. PMID: 32256370 Free PMC article.
-
Expanding Genotype-Phenotype Correlation of CLCNKA and CLCNKB Variants Linked to Hearing Loss.Int J Mol Sci. 2023 Dec 3;24(23):17077. doi: 10.3390/ijms242317077. Int J Mol Sci. 2023. PMID: 38069401 Free PMC article.
-
CD8+ T cells stimulate Na-Cl co-transporter NCC in distal convoluted tubules leading to salt-sensitive hypertension.Nat Commun. 2017 Jan 9;8:14037. doi: 10.1038/ncomms14037. Nat Commun. 2017. PMID: 28067240 Free PMC article.
References
-
- Adachi S., Uchida S., Ito H., Hata M., Hiroe M., Marumo F., Sasaki S. (1994). Two isoforms of a chloride channel predominantly expressed in thick ascending limb of Henle's loop and collecting ducts of rat kidney. J. Biol. Chem. 269, 17677–17683 - PubMed
-
- Alvarez O., Gonzalez C., Latorre R. (2002). Counting channels: a tutorial guide on ion channel fluctuation analysis. Adv. Physiol Educ. 26, 327–341 - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

