In vivo spike-timing-dependent plasticity in the optic tectum of Xenopus laevis
- PMID: 21423493
- PMCID: PMC3059697
- DOI: 10.3389/fnsyn.2010.00007
In vivo spike-timing-dependent plasticity in the optic tectum of Xenopus laevis
Abstract
Spike-timing-dependent plasticity (STDP) is found in vivo in a variety of systems and species, but the first demonstrations of in vivo STDP were carried out in the optic tectum of Xenopus laevis embryos. Since then, the optic tectum has served as an excellent experimental model for studying STDP in sensory systems, allowing researchers to probe the developmental consequences of this form of synaptic plasticity during early development. In this review, we will describe what is known about the role of STDP in shaping feed-forward and recurrent circuits in the optic tectum with a focus on the functional implications for vision. We will discuss both the similarities and differences between the optic tectum and mammalian sensory systems that are relevant to STDP. Finally, we will highlight the unique properties of the embryonic tectum that make it an important system for researchers who are interested in how STDP contributes to activity-dependent development of sensory computations.
Keywords: optic tectum; receptive field; spike-timing-dependent plasticity; synaptic development; visual system.
Figures

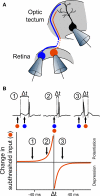

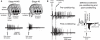
Similar articles
-
Does spike timing-dependent synaptic plasticity underlie memory formation?Clin Exp Pharmacol Physiol. 2007 Oct;34(10):1070-6. doi: 10.1111/j.1440-1681.2007.04724.x. Clin Exp Pharmacol Physiol. 2007. PMID: 17714096 Review.
-
Development and spike timing-dependent plasticity of recurrent excitation in the Xenopus optic tectum.Nat Neurosci. 2008 Apr;11(4):467-75. doi: 10.1038/nn2076. Epub 2008 Mar 23. Nat Neurosci. 2008. PMID: 18344990
-
Direct intertectal inputs are an integral component of the bilateral sensorimotor circuit for behavior in Xenopus tadpoles.J Neurophysiol. 2018 May 1;119(5):1947-1961. doi: 10.1152/jn.00051.2018. Epub 2018 Feb 14. J Neurophysiol. 2018. PMID: 29442555 Free PMC article.
-
Spike timing-dependent plasticity alters electrosensory neuron synaptic strength in vitro but does not consistently predict changes in sensory tuning in vivo.J Neurophysiol. 2023 May 1;129(5):1127-1144. doi: 10.1152/jn.00498.2022. Epub 2023 Apr 19. J Neurophysiol. 2023. PMID: 37073981 Free PMC article.
-
Spike timing-dependent plasticity of neural circuits.Neuron. 2004 Sep 30;44(1):23-30. doi: 10.1016/j.neuron.2004.09.007. Neuron. 2004. PMID: 15450157 Review.
Cited by
-
Binocular maps in Xenopus tectum: Visual experience and the development of isthmotectal topography.Dev Neurobiol. 2012 Apr;72(4):564-74. doi: 10.1002/dneu.20933. Dev Neurobiol. 2012. PMID: 21674812 Free PMC article. Review.
-
White Matter Plasticity Keeps the Brain in Tune: Axons Conduct While Glia Wrap.Front Cell Neurosci. 2018 Nov 16;12:428. doi: 10.3389/fncel.2018.00428. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30519159 Free PMC article. Review.
-
Questions about STDP as a General Model of Synaptic Plasticity.Front Synaptic Neurosci. 2010 Oct 4;2:140. doi: 10.3389/fnsyn.2010.00140. eCollection 2010. Front Synaptic Neurosci. 2010. PMID: 21423526 Free PMC article.
-
The applicability of spike time dependent plasticity to development.Front Synaptic Neurosci. 2010 Jul 19;2:30. doi: 10.3389/fnsyn.2010.00030. eCollection 2010. Front Synaptic Neurosci. 2010. PMID: 21423516 Free PMC article.
-
Spike-timing-dependent plasticity: a comprehensive overview.Front Synaptic Neurosci. 2012 Jul 12;4:2. doi: 10.3389/fnsyn.2012.00002. eCollection 2012. Front Synaptic Neurosci. 2012. PMID: 22807913 Free PMC article. No abstract available.
References
Grants and funding
LinkOut - more resources
Full Text Sources

