GABAergic activities control spike timing- and frequency-dependent long-term depression at hippocampal excitatory synapses
- PMID: 21423508
- PMCID: PMC3059709
- DOI: 10.3389/fnsyn.2010.00022
GABAergic activities control spike timing- and frequency-dependent long-term depression at hippocampal excitatory synapses
Abstract
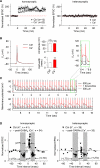
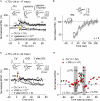
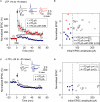
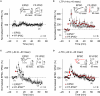
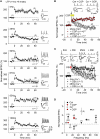
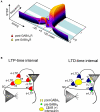
GABAergic interneuronal network activities in the hippocampus control a variety of neural functions, including learning and memory, by regulating θ and γ oscillations. How these GABAergic activities at pre- and postsynaptic sites of hippocampal CA1 pyramidal cells differentially contribute to synaptic function and plasticity during their repetitive pre- and postsynaptic spiking at θ and γ oscillations is largely unknown. We show here that activities mediated by postsynaptic GABA(A)Rs and presynaptic GABA(B)Rs determine, respectively, the spike timing- and frequency-dependence of activity-induced synaptic modifications at Schaffer collateral-CA1 excitatory synapses. We demonstrate that both feedforward and feedback GABA(A)R-mediated inhibition in the postsynaptic cell controls the spike timing-dependent long-term depression of excitatory inputs ("e-LTD") at the θ frequency. We also show that feedback postsynaptic inhibition specifically causes e-LTD of inputs that induce small postsynaptic currents (<70 pA) with LTP-timing, thus enforcing the requirement of cooperativity for induction of long-term potentiation at excitatory inputs ("e-LTP"). Furthermore, under spike-timing protocols that induce e-LTP and e-LTD at excitatory synapses, we observed parallel induction of LTP and LTD at inhibitory inputs ("i-LTP" and "i-LTD") to the same postsynaptic cells. Finally, we show that presynaptic GABA(B)R-mediated inhibition plays a major role in the induction of frequency-dependent e-LTD at α and β frequencies. These observations demonstrate the critical influence of GABAergic interneuronal network activities in regulating the spike timing- and frequency-dependences of long-term synaptic modifications in the hippocampus.
Keywords: CA1; GABA; STDP; frequency; hippocampal oscillation; spike timing.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

