Transcranial magnetic stimulation provides means to assess cortical plasticity and excitability in humans with fragile x syndrome and autism spectrum disorder
- PMID: 21423512
- PMCID: PMC3059673
- DOI: 10.3389/fnsyn.2010.00026
Transcranial magnetic stimulation provides means to assess cortical plasticity and excitability in humans with fragile x syndrome and autism spectrum disorder
Abstract
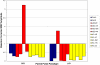
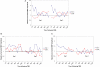
Fragile X Syndrome (FXS) is the most common heritable cause of intellectual disability. In vitro electrophysiologic data from mouse models of FXS suggest that loss of fragile X mental retardation protein affects intracortical excitability and synaptic plasticity. Specifically, the cortex appears hyperexcitable, and use-dependent long-term potentiation (LTP) and long-term depression (LTD) of synaptic strength are abnormal. Though animal models provide important information, FXS and other neurodevelopmental disorders are human diseases and as such translational research to evaluate cortical excitability and plasticity must be applied in the human. Transcranial magnetic stimulation paradigms have recently been developed to non-invasively investigate cortical excitability using paired pulse stimulation, as well as LTP- and LTD-like synaptic plasticity in response to theta burst stimulation (TBS) in vivo in the human. TBS applied on consecutive days can be used to measure metaplasticity (the ability of the synapse to undergo a second plastic change following a recent induction of plasticity). The current study investigated intracortical inhibition, plasticity and metaplasticity in full mutation females with FXS, participants with autism spectrum disorders (ASD), and neurotypical controls. Results suggest that intracortical inhibition is normal in participants with FXS, while plasticity and metaplasticity appear abnormal. ASD participants showed abnormalities in plasticity and metaplasticity, as well as heterogeneity in intracortical inhibition. Our findings highlight the utility of non-invasive neurophysiological measures to translate insights from animal models to humans with neurodevelopmental disorders, and thus provide direct confirmation of cortical dysfunction in patients with FXS and ASD.
Keywords: autism spectrum disorders; excitability; fragile X syndrome; paired pulse stimulation; plasticity; theta burst stimulation; transcranial magnetic stimulation.
Figures


Similar articles
-
Abnormal Mechanisms of Plasticity and Metaplasticity in Autism Spectrum Disorders and Fragile X Syndrome.J Child Adolesc Psychopharmacol. 2016 Sep;26(7):617-24. doi: 10.1089/cap.2015.0166. Epub 2016 May 24. J Child Adolesc Psychopharmacol. 2016. PMID: 27218148 Free PMC article.
-
TMS: using the theta-burst protocol to explore mechanism of plasticity in individuals with Fragile X syndrome and autism.J Vis Exp. 2010 Dec 28;(46):2272. doi: 10.3791/2272. J Vis Exp. 2010. PMID: 21248685 Free PMC article.
-
Investigations of motor-cortex cortical plasticity following facilitatory and inhibitory transcranial theta-burst stimulation in schizophrenia: a proof-of-concept study.J Psychiatr Res. 2015 Feb;61:196-204. doi: 10.1016/j.jpsychires.2014.12.006. Epub 2014 Dec 19. J Psychiatr Res. 2015. PMID: 25555304
-
Mechanism-based treatments in neurodevelopmental disorders: fragile X syndrome.Pediatr Neurol. 2014 Apr;50(4):297-302. doi: 10.1016/j.pediatrneurol.2013.12.001. Epub 2013 Dec 4. Pediatr Neurol. 2014. PMID: 24518745 Review.
-
Melatonin as a Novel Interventional Candidate for Fragile X Syndrome with Autism Spectrum Disorder in Humans.Int J Mol Sci. 2017 Jun 20;18(6):1314. doi: 10.3390/ijms18061314. Int J Mol Sci. 2017. PMID: 28632163 Free PMC article. Review.
Cited by
-
Characterizing brain cortical plasticity and network dynamics across the age-span in health and disease with TMS-EEG and TMS-fMRI.Brain Topogr. 2011 Oct;24(3-4):302-15. doi: 10.1007/s10548-011-0196-8. Epub 2011 Aug 14. Brain Topogr. 2011. PMID: 21842407 Free PMC article.
-
Use of transcranial magnetic stimulation in autism spectrum disorders.J Autism Dev Disord. 2015 Feb;45(2):524-36. doi: 10.1007/s10803-013-1960-2. J Autism Dev Disord. 2015. PMID: 24127165 Free PMC article. Review.
-
rTMS neuromodulation improves electrocortical functional measures of information processing and behavioral responses in autism.Front Syst Neurosci. 2014 Aug 6;8:134. doi: 10.3389/fnsys.2014.00134. eCollection 2014. Front Syst Neurosci. 2014. PMID: 25147508 Free PMC article.
-
The relationship between brain oscillatory activity and therapeutic effectiveness of transcranial magnetic stimulation in the treatment of major depressive disorder.Front Hum Neurosci. 2013 Feb 26;7:37. doi: 10.3389/fnhum.2013.00037. eCollection 2013. Front Hum Neurosci. 2013. PMID: 23550274 Free PMC article.
-
Effect of 30 Hz theta burst transcranial magnetic stimulation on the primary motor cortex in children and adolescents.Front Hum Neurosci. 2015 Feb 25;9:91. doi: 10.3389/fnhum.2015.00091. eCollection 2015. Front Hum Neurosci. 2015. PMID: 25762919 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

