A Ca-Based Computational Model for NMDA Receptor-Dependent Synaptic Plasticity at Individual Post-Synaptic Spines in the Hippocampus
- PMID: 21423517
- PMCID: PMC3059685
- DOI: 10.3389/fnsyn.2010.00031
A Ca-Based Computational Model for NMDA Receptor-Dependent Synaptic Plasticity at Individual Post-Synaptic Spines in the Hippocampus
Abstract
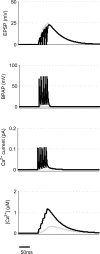
Associative synaptic plasticity is synapse specific and requires coincident activity in pre-synaptic and post-synaptic neurons to activate NMDA receptors (NMDARs). The resultant Ca(2+) influx is the critical trigger for the induction of synaptic plasticity. Given its centrality for the induction of synaptic plasticity, a model for NMDAR activation incorporating the timing of pre-synaptic glutamate release and post-synaptic depolarization by back-propagating action potentials could potentially predict the pre- and post-synaptic spike patterns required to induce synaptic plasticity. We have developed such a model by incorporating currently available data on the timecourse and amplitude of the post-synaptic membrane potential within individual spines. We couple this with data on the kinetics of synaptic NMDARs and then use the model to predict the continuous spine [Ca(2+)] in response to regular or irregular pre- and post-synaptic spike patterns. We then incorporate experimental data from synaptic plasticity induction protocols by regular activity patterns to couple the predicted local peak [Ca(2+)] to changes in synaptic strength. We find that our model accurately describes [Ca(2+)] in dendritic spines resulting from NMDAR activation during pre-synaptic and post-synaptic activity when compared to previous experimental observations. The model also replicates the experimentally determined plasticity outcome of regular and irregular spike patterns when applied to a single synapse. This model could therefore be used to predict the induction of synaptic plasticity under a variety of experimental conditions and spike patterns.
Keywords: NMDA receptor; dendritic spines; hippocampus; spike timing-dependent plasticity; synaptic plasticity.
Figures





References
-
- Bashir Z. I., Bortolotto Z. A., Davies C. H., Berretta N., Irving A. J., Seal A. J., Henley J. M., Jane D. E., Watkins J. C., Collingridge G. L. (1993). Induction of LTP in the hippocampus needs synaptic activation of glutamate metabotropic receptors. Nature 363, 347–35010.1038/363347a0 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

