Experience-dependent changes in excitatory and inhibitory receptor subunit expression in visual cortex
- PMID: 21423524
- PMCID: PMC3059668
- DOI: 10.3389/fnsyn.2010.00138
Experience-dependent changes in excitatory and inhibitory receptor subunit expression in visual cortex
Abstract
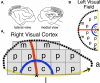
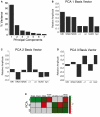
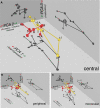
Experience-dependent development of visual cortex depends on the balance between excitatory and inhibitory activity. This activity is regulated by key excitatory (NMDA, AMPA) and inhibitory (GABA(A)) receptors. The composition of these receptors changes developmentally, affecting the excitatory-inhibitory (E/I) balance and synaptic plasticity. Until now, it has been unclear how abnormal visual experience affects this balance. To examine this question, we measured developmental changes in excitatory and inhibitory receptor subunits in visual cortex following normal visual experience and monocular deprivation. We used Western blot analysis to quantify expression of excitatory (NR1, NR2A, NR2B, GluR2) and inhibitory (GABA(A)α1, GABA(A)α3) receptor subunits. Monocular deprivation promoted a complex pattern of changes in receptor subunit expression that varied with age and was most severe in the region of visual cortex representing the central visual field. To characterize the multidimensional pattern of experience-dependent change in these synaptic mechanisms, we applied a neuroinformatics approach using principal component analysis. We found that monocular deprivation (i) causes a large portion of the normal developmental trajectory to be bypassed, (ii) shifts the E/I balance in favor of more inhibition, and (iii) accelerates the maturation of receptor subunits. Taken together, these results show that monocularly deprived animals have an abnormal balance of the synaptic machinery needed for functional maturation of cortical circuits and for developmental plasticity. This raises the possibility that interventions intended to treat amblyopia may need to address multiple synaptic mechanisms to produce optimal recovery.
Keywords: AMPA; GABAA; NMDA; amblyopia; development; excitatory–inhibitory balance; monocular deprivation; plasticity.
Figures







Similar articles
-
Two forms of synaptic plasticity with distinct dependence on age, experience, and NMDA receptor subtype in rat visual cortex.J Neurosci. 2003 Jul 23;23(16):6557-66. doi: 10.1523/JNEUROSCI.23-16-06557.2003. J Neurosci. 2003. PMID: 12878697 Free PMC article.
-
Postnatal development of NR1, NR2A and NR2B immunoreactivity in the visual cortex of the rat.Brain Res. 2000 Mar 17;859(1):26-37. doi: 10.1016/s0006-8993(99)02450-6. Brain Res. 2000. PMID: 10720612
-
AMPA glutamate receptor subunit 2 in normal and visually deprived macaque visual cortex.Vis Neurosci. 2002 Sep-Oct;19(5):563-73. doi: 10.1017/s0952523802195022. Vis Neurosci. 2002. PMID: 12507323
-
Critical period mechanisms in developing visual cortex.Curr Top Dev Biol. 2005;69:215-37. doi: 10.1016/S0070-2153(05)69008-4. Curr Top Dev Biol. 2005. PMID: 16243601 Review.
-
Molecular mechanisms that underlie structural and functional changes at the postsynaptic membrane during synaptic plasticity.Prog Neurobiol. 1998 Aug;55(6):611-40. doi: 10.1016/s0301-0082(98)00026-4. Prog Neurobiol. 1998. PMID: 9670221 Review.
Cited by
-
Experience-Dependent Changes in Myelin Basic Protein Expression in Adult Visual and Somatosensory Cortex.Front Cell Neurosci. 2020 Mar 17;14:56. doi: 10.3389/fncel.2020.00056. eCollection 2020. Front Cell Neurosci. 2020. PMID: 32265660 Free PMC article.
-
A Primer on Constructing Plasticity Phenotypes to Classify Experience-Dependent Development of the Visual Cortex.Front Cell Neurosci. 2020 Aug 27;14:245. doi: 10.3389/fncel.2020.00245. eCollection 2020. Front Cell Neurosci. 2020. PMID: 33192303 Free PMC article.
-
Developmental effect of light deprivation on synaptic plasticity of rats' hippocampus: implications for melatonin.Iran J Basic Med Sci. 2016 Aug;19(8):899-909. Iran J Basic Med Sci. 2016. PMID: 27746873 Free PMC article.
-
Characterizing synaptic protein development in human visual cortex enables alignment of synaptic age with rat visual cortex.Front Neural Circuits. 2015 Feb 12;9:3. doi: 10.3389/fncir.2015.00003. eCollection 2015. Front Neural Circuits. 2015. PMID: 25729353 Free PMC article.
-
Development of Glutamatergic Proteins in Human Visual Cortex across the Lifespan.J Neurosci. 2017 Jun 21;37(25):6031-6042. doi: 10.1523/JNEUROSCI.2304-16.2017. Epub 2017 May 29. J Neurosci. 2017. PMID: 28554889 Free PMC article.
References
-
- Bonnert T. P., McKernan R. M., Farrar S., le Bourdelles B., Heavens R. P., Smith D. W., Hewson L., Rigby M. R., Sirinathsinghji D. J., Brown N., Wafford K. A., Whiting P. J. (1999). Theta, a novel gamma-aminobutyric acid type A receptor subunit. Proc. Natl. Acad. Sci. U.S.A. 96, 9891–989610.1073/pnas.96.17.9891 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

