Does observation of postural imbalance induce a postural reaction?
- PMID: 21423622
- PMCID: PMC3057996
- DOI: 10.1371/journal.pone.0017799
Does observation of postural imbalance induce a postural reaction?
Erratum in
- PLoS One. 2011;6(3). doi: 10.1371/annotation/aff2c1f3-08af-4a36-bf53-0df2bfd9c320
Abstract
Background: Several studies bring evidence that action observation elicits contagious responses during social interactions. However automatic imitative tendencies are generally inhibited and it remains unclear in which conditions mere action observation triggers motor behaviours. In this study, we addressed the question of contagious postural responses when observing human imbalance.
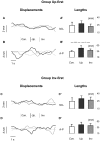
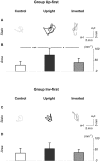
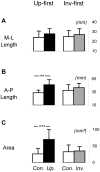
Methodology/principal findings: We recorded participants' body sway while they observed a fixation cross (control condition), an upright point-light display of a gymnast balancing on a rope, and the same point-light display presented upside down. Our results showed that, when the upright stimulus was displayed prior to the inverted one, centre of pressure area and antero-posterior path length were significantly greater in the upright condition compared to the control and upside down conditions.
Conclusions/significance: These results demonstrate a contagious postural reaction suggesting a partial inefficiency of inhibitory processes. Further, kinematic information was sufficient to trigger this reaction. The difference recorded between the upright and upside down conditions indicates that the contagion effect was dependent on the integration of gravity constraints by body kinematics. Interestingly, the postural response was sensitive to habituation, and seemed to disappear when the observer was previously shown an inverted display. The motor contagion recorded here is consistent with previous work showing vegetative output during observation of an effortful movement and could indicate that lower level control facilitates contagion effects.
Conflict of interest statement
Figures
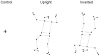
Similar articles
-
Postural Stabilization Strategies to Motor Contagion Induced by Action Observation Are Impaired in Parkinson's Disease.Front Neurol. 2018 Mar 1;9:105. doi: 10.3389/fneur.2018.00105. eCollection 2018. Front Neurol. 2018. PMID: 29545771 Free PMC article.
-
Do equilibrium constraints modulate postural reaction when viewing imbalance?Brain Cogn. 2012 Jul;79(2):89-95. doi: 10.1016/j.bandc.2012.02.008. Epub 2012 Mar 30. Brain Cogn. 2012. PMID: 22466502
-
The development of infant upright posture: sway less or sway differently?Exp Brain Res. 2008 Mar;186(2):293-303. doi: 10.1007/s00221-007-1236-1. Epub 2007 Dec 5. Exp Brain Res. 2008. PMID: 18057920
-
Structural changes in postural sway lend insight into effects of balance training, vision, and support surface on postural control in a healthy population.Eur J Appl Physiol. 2011 Jul;111(7):1485-95. doi: 10.1007/s00421-010-1770-6. Epub 2010 Dec 17. Eur J Appl Physiol. 2011. PMID: 21165641 Clinical Trial.
-
Interpersonal interactions for haptic guidance during balance exercises.Gait Posture. 2018 Sep;65:129-136. doi: 10.1016/j.gaitpost.2018.07.163. Epub 2018 Jul 20. Gait Posture. 2018. PMID: 30558919
Cited by
-
A Russian Adaptation of the Emotional Contagion Scale.Front Psychol. 2022 Aug 17;13:872718. doi: 10.3389/fpsyg.2022.872718. eCollection 2022. Front Psychol. 2022. PMID: 36059773 Free PMC article.
-
A new methodical approach in neuroscience: assessing inter-personal brain coupling using functional near-infrared imaging (fNIRI) hyperscanning.Front Hum Neurosci. 2013 Nov 27;7:813. doi: 10.3389/fnhum.2013.00813. Front Hum Neurosci. 2013. PMID: 24348362 Free PMC article. Review.
-
Cerebral Dynamics during the Observation of Point-Light Displays Depicting Postural Adjustments.Front Hum Neurosci. 2017 May 8;11:217. doi: 10.3389/fnhum.2017.00217. eCollection 2017. Front Hum Neurosci. 2017. PMID: 28533748 Free PMC article.
-
Postural Stabilization Strategies to Motor Contagion Induced by Action Observation Are Impaired in Parkinson's Disease.Front Neurol. 2018 Mar 1;9:105. doi: 10.3389/fneur.2018.00105. eCollection 2018. Front Neurol. 2018. PMID: 29545771 Free PMC article.
-
The modulation of motor control by imitating non-biological motions: a study about motor resonance.J Phys Ther Sci. 2018 Jan;30(1):159-163. doi: 10.1589/jpts.30.159. Epub 2018 Jan 27. J Phys Ther Sci. 2018. PMID: 29410589 Free PMC article.
References
-
- Singer T. The neuronal basis and ontogeny of empathy and mind reading: review of literature and implications for future research. Neurosci Biobehav Rev. 2006;30:855–863. - PubMed
-
- Brass M, Bekkering H, Wohlschläger A, Prinz W. Compatibility between observed and executed finger movements: comparing symbolic, spatial, and imitative cues. Brain Cogn. 2000;44:124–143. - PubMed
-
- Kilner JM, Paulignan Y, Blakemore SJ. An interference effect of observed biological movement on action. Curr Biol. 2003;13:522–525. - PubMed
-
- Bove M, Tacchino A, Pelosin E, Moisello C, Abbruzzese G, et al. Spontaneous movement tempo is influenced by observation of rhythmical actions. Brain Research Bulletin. 2009;80:122–127. - PubMed
-
- Watanabe K. Behavioral speed contagion: automatic modulation of movement timing by observation of body movements. Cognition. 2008;106:1514–1524. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

