Design of glycopeptides used to investigate class II MHC binding and T-cell responses associated with autoimmune arthritis
- PMID: 21423632
- PMCID: PMC3058040
- DOI: 10.1371/journal.pone.0017881
Design of glycopeptides used to investigate class II MHC binding and T-cell responses associated with autoimmune arthritis
Abstract
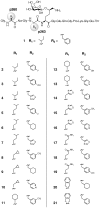
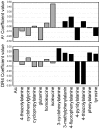
The glycopeptide fragment CII259-273 from type II collagen (CII) binds to the murine A(q) and human DR4 class II Major Histocompatibility Complex (MHC II) proteins, which are associated with development of murine collagen-induced arthritis (CIA) and rheumatoid arthritis (RA), respectively. It has been shown that CII259-273 can be used in therapeutic vaccination of CIA. This glycopeptide also elicits responses from T-cells obtained from RA patients, which indicates that it has an important role in RA as well. We now present a methodology for studies of (glyco)peptide-receptor interactions based on a combination of structure-based virtual screening, ligand-based statistical molecular design and biological evaluations. This methodology included the design of a CII259-273 glycopeptide library in which two anchor positions crucial for binding in pockets of A(q) and DR4 were varied. Synthesis and biological evaluation of the designed glycopeptides provided novel structure-activity relationship (SAR) understanding of binding to A(q) and DR4. Glycopeptides that retained high affinities for these MHC II proteins and induced strong responses in panels of T-cell hybridomas were also identified. An analysis of all the responses revealed groups of glycopeptides with different response patterns that are of high interest for vaccination studies in CIA. Moreover, the SAR understanding obtained in this study provides a platform for the design of second-generation glycopeptides with tuned MHC affinities and T-cell responses.
Conflict of interest statement
Figures




Similar articles
-
Oxazole-modified glycopeptides that target arthritis-associated class II MHC A(q) and DR4 proteins.Org Biomol Chem. 2010 Jun 28;8(13):2931-40. doi: 10.1039/c003640d. Epub 2010 May 20. Org Biomol Chem. 2010. PMID: 20485848
-
Probing molecular interactions within class II MHC Aq/glycopeptide/T-cell receptor complexes associated with collagen-induced arthritis.J Med Chem. 2007 Nov 15;50(23):5627-43. doi: 10.1021/jm0705410. Epub 2007 Oct 18. J Med Chem. 2007. PMID: 17944452
-
Quantitative structure-activity relationship of peptides binding to the class II major histocompatibility complex molecule Aq associated with autoimmune arthritis.J Med Chem. 2007 May 3;50(9):2049-59. doi: 10.1021/jm061209b. Epub 2007 Apr 11. J Med Chem. 2007. PMID: 17425295
-
Immunopathogenesis of collagen arthritis.Springer Semin Immunopathol. 2003 Aug;25(1):3-18. doi: 10.1007/s00281-003-0127-1. Springer Semin Immunopathol. 2003. PMID: 12904888 Review.
-
Synthetic methods of glycopeptide assembly, and biological analysis of glycopeptide products.Curr Opin Chem Biol. 1997 Dec;1(4):552-63. doi: 10.1016/s1367-5931(97)80052-x. Curr Opin Chem Biol. 1997. PMID: 9667891 Review.
Cited by
-
Glycosylation modulates melanoma cell α2β1 and α3β1 integrin interactions with type IV collagen.J Biol Chem. 2014 Aug 1;289(31):21591-604. doi: 10.1074/jbc.M114.572073. Epub 2014 Jun 23. J Biol Chem. 2014. PMID: 24958723 Free PMC article.
-
Collagenolytic Matrix Metalloproteinase Activities toward Peptomeric Triple-Helical Substrates.Biochemistry. 2015 May 19;54(19):3110-21. doi: 10.1021/acs.biochem.5b00110. Epub 2015 May 5. Biochemistry. 2015. PMID: 25897652 Free PMC article.
-
Matched Peptides: Tuning Matched Molecular Pair Analysis for Biopharmaceutical Applications.J Chem Inf Model. 2015 Nov 23;55(11):2315-23. doi: 10.1021/acs.jcim.5b00476. Epub 2015 Nov 6. J Chem Inf Model. 2015. PMID: 26501781 Free PMC article.
-
Enhanced epimerization of glycosylated amino acids during solid-phase peptide synthesis.J Am Chem Soc. 2012 Apr 11;134(14):6316-25. doi: 10.1021/ja212188r. Epub 2012 Mar 27. J Am Chem Soc. 2012. PMID: 22390544 Free PMC article.
-
Korean red ginseng saponin fraction rich in ginsenoside-Rb1, Rc and Rb2 attenuates the severity of mouse collagen-induced arthritis.Mediators Inflamm. 2014;2014:748964. doi: 10.1155/2014/748964. Epub 2014 Apr 16. Mediators Inflamm. 2014. PMID: 24833816 Free PMC article.
References
-
- Gregersen PK, Silver J, Winchester RJ. The Shared Epitope Hypothesis. An Approach to Understanding the Molecular Genetics of Susceptibility to Rheumatoid Arthritis. Arthritis Rheum. 1987;30:1205–1213. - PubMed
-
- Courtenay JS, Dallman MJ, Dayan AD, Martin A, Mosedale B. Immunisation Against Heterologous Type II Collagen Induces Arthritis in Mice. Nature. 1980;283:666–668. - PubMed
-
- Brunsberg U, Gustafsson K, Jansson L, Michaëlsson E, Ährlund-Richter L, et al. Expression of a Transgenic Class II Ab Gene Confers Susceptibility to Collagen-Induced Arthritis. Eur J Immunol. 1994;24:1698–1702. - PubMed
-
- Bäcklund J, Treschow A, Bockermann R, Holm B, Holm L, et al. Glycosylation of Type II Collagen is of Major Importance for T Cell Tolerance and Pathology in Collagen-Induced Arthritis. Eur J Immunol. 2002;32:3776–3784. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

