Cryptic patterning of avian skin confers a developmental facility for loss of neck feathering
- PMID: 21423653
- PMCID: PMC3057954
- DOI: 10.1371/journal.pbio.1001028
Cryptic patterning of avian skin confers a developmental facility for loss of neck feathering
Abstract
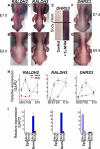
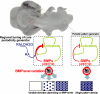
Vertebrate skin is characterized by its patterned array of appendages, whether feathers, hairs, or scales. In avian skin the distribution of feathers occurs on two distinct spatial levels. Grouping of feathers within discrete tracts, with bare skin lying between the tracts, is termed the macropattern, while the smaller scale periodic spacing between individual feathers is referred to as the micropattern. The degree of integration between the patterning mechanisms that operate on these two scales during development and the mechanisms underlying the remarkable evolvability of skin macropatterns are unknown. A striking example of macropattern variation is the convergent loss of neck feathering in multiple species, a trait associated with heat tolerance in both wild and domestic birds. In chicken, a mutation called Naked neck is characterized by a reduction of body feathering and completely bare neck. Here we perform genetic fine mapping of the causative region and identify a large insertion associated with the Naked neck trait. A strong candidate gene in the critical interval, BMP12/GDF7, displays markedly elevated expression in Naked neck embryonic skin due to a cis-regulatory effect of the causative mutation. BMP family members inhibit embryonic feather formation by acting in a reaction-diffusion mechanism, and we find that selective production of retinoic acid by neck skin potentiates BMP signaling, making neck skin more sensitive than body skin to suppression of feather development. This selective production of retinoic acid by neck skin constitutes a cryptic pattern as its effects on feathering are not revealed until gross BMP levels are altered. This developmental modularity of neck and body skin allows simple quantitative changes in BMP levels to produce a sparsely feathered or bare neck while maintaining robust feather patterning on the body.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




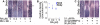
Comment in
-
How bird necks get naked.PLoS Biol. 2011 Mar;9(3):e1001029. doi: 10.1371/journal.pbio.1001029. Epub 2011 Mar 15. PLoS Biol. 2011. PMID: 21423651 Free PMC article. No abstract available.
References
-
- Olivera-Martinez I, Viallet J. P, Michon F, Pearton D. J, Dhouailly D. The different steps of skin formation in vertebrates. Int J Dev Biol. 2004;48:107–115. - PubMed
-
- Fliniaux I, Viallet J. P, Dhouailly D. Signaling dynamics of feather tract formation from the chick somatopleure. Development. 2004;131:3955–3966. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

