A rigidifying salt-bridge favors the activity of thermophilic enzyme at high temperatures at the expense of low-temperature activity
- PMID: 21423654
- PMCID: PMC3057955
- DOI: 10.1371/journal.pbio.1001027
A rigidifying salt-bridge favors the activity of thermophilic enzyme at high temperatures at the expense of low-temperature activity
Abstract
Background: Thermophilic enzymes are often less active than their mesophilic homologues at low temperatures. One hypothesis to explain this observation is that the extra stabilizing interactions increase the rigidity of thermophilic enzymes and hence reduce their activity. Here we employed a thermophilic acylphosphatase from Pyrococcus horikoshii and its homologous mesophilic acylphosphatase from human as a model to study how local rigidity of an active-site residue affects the enzymatic activity.
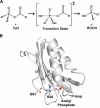
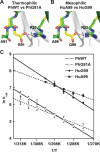
Methods and findings: Acylphosphatases have a unique structural feature that its conserved active-site arginine residue forms a salt-bridge with the C-terminal carboxyl group only in thermophilic acylphosphatases, but not in mesophilic acylphosphatases. We perturbed the local rigidity of this active-site residue by removing the salt-bridge in the thermophilic acylphosphatase and by introducing the salt-bridge in the mesophilic homologue. The mutagenesis design was confirmed by x-ray crystallography. Removing the salt-bridge in the thermophilic enzyme lowered the activation energy that decreased the activation enthalpy and entropy. Conversely, the introduction of the salt-bridge to the mesophilic homologue increased the activation energy and resulted in increases in both activation enthalpy and entropy. Revealed by molecular dynamics simulations, the unrestrained arginine residue can populate more rotamer conformations, and the loss of this conformational freedom upon the formation of transition state justified the observed reduction in activation entropy.
Conclusions: Our results support the conclusion that restricting the active-site flexibility entropically favors the enzymatic activity at high temperatures. However, the accompanying enthalpy-entropy compensation leads to a stronger temperature-dependency of the enzymatic activity, which explains the less active nature of the thermophilic enzymes at low temperatures.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Georlette D, Damien B, Blaise V, Depiereux E, Uversky V. N, et al. Structural and functional adaptations to extreme temperatures in psychrophilic, mesophilic, and thermophilic DNA ligases. J Biol Chem. 2003;278:37015–37023. - PubMed
-
- Bae E, Phillips G. N. Structures and analysis of highly homologous psychrophilic, mesophilic, and thermophilic adenylate kinases. J Biol Chem. 2004;279:28202–28208. - PubMed
-
- Collins T, Meuwis M. A, Gerday C, Feller G. Activity, stability and flexibility in glycosidases adapted to extreme thermal environments. J Mol Biol. 2003;328:419–428. - PubMed
-
- Olufsen M, Smalas A. O, Moe E, Brandsdal B. O. Increased flexibility as a strategy for cold adaptation: a comparative molecular dynamics study of cold- and warm-active uracil DNA glycosylase. J Biol Chem. 2005;280:18042–18048. - PubMed
-
- D'Amico S, Sohier J. S, Feller G. Kinetics and energetics of ligand binding determined by microcalorimetry: insights into active site mobility in a psychrophilic alpha-amylase. J Mol Biol. 2006;358:1296–1304. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

