BAG3 directly interacts with mutated alphaB-crystallin to suppress its aggregation and toxicity
- PMID: 21423662
- PMCID: PMC3057972
- DOI: 10.1371/journal.pone.0016828
BAG3 directly interacts with mutated alphaB-crystallin to suppress its aggregation and toxicity
Abstract
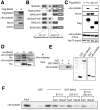
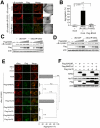
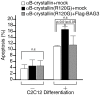
A homozygous disruption or genetic mutation of the bag3 gene causes progressive myofibrillar myopathy in mouse and human skeletal and cardiac muscle disorder while mutations in the small heat shock protein αB-crystallin gene (CRYAB) are reported to be responsible for myofibrillar myopathy. Here, we demonstrate that BAG3 directly binds to wild-type αB-crystallin and the αB-crystallin mutant R120G, via the intermediate domain of BAG3. Peptides that inhibit this interaction in an in vitro binding assay indicate that two conserved Ile-Pro-Val regions of BAG3 are involved in the interaction with αB-crystallin, which is similar to results showing BAG3 binding to HspB8 and HspB6. BAG3 overexpression increased αB-crystallin R120G solubility and inhibited its intracellular aggregation in HEK293 cells. BAG3 suppressed cell death induced by αB-crystallin R120G overexpression in differentiating C2C12 mouse myoblast cells. Our findings indicate a novel function for BAG3 in inhibiting protein aggregation caused by the genetic mutation of CRYAB responsible for human myofibrillar myopathy.
Conflict of interest statement
Figures

Similar articles
-
Crystal structure of R120G disease mutant of human αB-crystallin domain dimer shows closure of a groove.J Mol Biol. 2011 Apr 22;408(1):118-34. doi: 10.1016/j.jmb.2011.02.020. Epub 2011 Feb 15. J Mol Biol. 2011. PMID: 21329698 Free PMC article.
-
Single molecule force spectroscopy of the cardiac titin N2B element: effects of the molecular chaperone alphaB-crystallin with disease-causing mutations.J Biol Chem. 2009 May 15;284(20):13914-13923. doi: 10.1074/jbc.M809743200. Epub 2009 Mar 12. J Biol Chem. 2009. PMID: 19282282 Free PMC article.
-
Identification of the key structural motifs involved in HspB8/HspB6-Bag3 interaction.Biochem J. 2009 Dec 14;425(1):245-55. doi: 10.1042/BJ20090907. Biochem J. 2009. PMID: 19845507
-
Functional sequences in human alphaB crystallin.Biochim Biophys Acta. 2016 Jan;1860(1 Pt B):240-5. doi: 10.1016/j.bbagen.2015.08.014. Epub 2015 Sep 2. Biochim Biophys Acta. 2016. PMID: 26341790 Free PMC article. Review.
-
Dynamical structure of αB-crystallin.Prog Biophys Mol Biol. 2014 Jul;115(1):11-20. doi: 10.1016/j.pbiomolbio.2014.03.003. Epub 2014 Mar 24. Prog Biophys Mol Biol. 2014. PMID: 24674783 Review.
Cited by
-
Small heat shock proteins in redox metabolism: implications for cardiovascular diseases.Int J Biochem Cell Biol. 2012 Oct;44(10):1632-45. doi: 10.1016/j.biocel.2012.06.006. Epub 2012 Jun 15. Int J Biochem Cell Biol. 2012. PMID: 22710345 Free PMC article. Review.
-
Genetic cardiomyopathies. Lessons learned from humans, mice, and zebrafish.Herz. 2012 Sep;37(6):612-7. doi: 10.1007/s00059-012-3651-8. Herz. 2012. PMID: 22767018 Review.
-
NFκB is a central regulator of protein quality control in response to protein aggregation stresses via autophagy modulation.Mol Biol Cell. 2016 Jun 1;27(11):1712-27. doi: 10.1091/mbc.E15-12-0835. Epub 2016 Apr 13. Mol Biol Cell. 2016. PMID: 27075172 Free PMC article.
-
BAG3 Is a Modular, Scaffolding Protein that physically Links Heat Shock Protein 70 (Hsp70) to the Small Heat Shock Proteins.J Mol Biol. 2017 Jan 6;429(1):128-141. doi: 10.1016/j.jmb.2016.11.013. Epub 2016 Nov 21. J Mol Biol. 2017. PMID: 27884606 Free PMC article.
-
Novel BAG3 Variants in African American Patients With Cardiomyopathy: Reduced β-Adrenergic Responsiveness in Excitation-Contraction.J Card Fail. 2020 Dec;26(12):1075-1085. doi: 10.1016/j.cardfail.2020.09.009. Epub 2020 Sep 18. J Card Fail. 2020. PMID: 32956817 Free PMC article.
References
-
- Knoll R, Hoshijima M, Hoffman HM, Person V, Lorenzen-Schmidt I, et al. The cardiac mechanical stretch sensor machinery involves a Z disc complex that is defective in a subset of human dilated cardiomyopathy. Cell. 2002;111:943–955. - PubMed
-
- Engel AG. Myofibrillar myopathy. Ann Neurol. 1999;46:681–683. - PubMed
-
- Vicart P, Caron A, Guicheney P, Li Z, Prevost MC, et al. A missense mutation in the alphaB-crystallin chaperone gene causes a desmin-related myopathy. Nat Genet. 1998;20:92–95. - PubMed
-
- Selcen D, Engel AG. Myofibrillar myopathy caused by novel dominant negative alpha B-crystallin mutations. Ann Neurol. 2003;54:804–810. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

