The moving junction protein RON8 facilitates firm attachment and host cell invasion in Toxoplasma gondii
- PMID: 21423671
- PMCID: PMC3053350
- DOI: 10.1371/journal.ppat.1002007
The moving junction protein RON8 facilitates firm attachment and host cell invasion in Toxoplasma gondii
Abstract
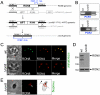
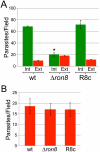
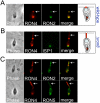
The apicomplexan moving junction (MJ) is a highly conserved structure formed during host cell entry that anchors the invading parasite to the host cell and serves as a molecular sieve of host membrane proteins that protects the parasitophorous vacuole from host lysosomal destruction. While recent work in Toxoplasma and Plasmodium has reinforced the composition of the MJ as an important association of rhoptry neck proteins (RONs) with micronemal AMA1, little is known of the precise role of RONs in the junction or how they are targeted to the neck subcompartment. We report the first functional analysis of a MJ/RON protein by disrupting RON8 in T. gondii. Parasites lacking RON8 are severely impaired in both attachment and invasion, indicating that RON8 enables the parasite to establish a firm clasp on the host cell and commit to invasion. The remaining junction components frequently drag in trails behind invading knockout parasites and illustrate a malformed complex without RON8. Complementation of Δron8 parasites restores invasion and reveals a processing event at the RON8 C-terminus. Replacement of an N-terminal region of RON8 with a mCherry reporter separates regions within RON8 that are necessary for rhoptry targeting and complex formation from those required for function during invasion. Finally, the invasion defects in Δron8 parasites seen in vitro translate to radically impaired virulence in infected mice, promoting a model in which RON8 has a crucial and unprecedented task in committing Toxoplasma to host cell entry.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Baum J, Richard D, Healer J, Rug M, Krnajski Z, et al. A conserved molecular motor drives cell invasion and gliding motility across malaria life cycle stages and other apicomplexan parasites. J Biol Chem. 2006;281:5197–5208. - PubMed
-
- Cowman AF, Crabb BS. Invasion of red blood cells by malaria parasites. Cell. 2006;124:755–766. - PubMed
-
- Carruthers V, Boothroyd JC. Pulling together: an integrated model of Toxoplasma cell invasion. Curr Opin Microbiol. 2007;10:83–89. - PubMed
-
- Soldati-Favre D. Molecular dissection of host cell invasion by the apicomplexans: the glideosome. Parasite. 2008;15:197–205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

