Longistatin, a plasminogen activator, is key to the availability of blood-meals for ixodid ticks
- PMID: 21423674
- PMCID: PMC3053353
- DOI: 10.1371/journal.ppat.1001312
Longistatin, a plasminogen activator, is key to the availability of blood-meals for ixodid ticks
Abstract
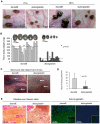
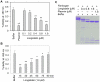
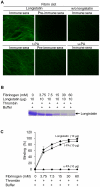
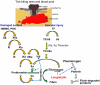
Ixodid ticks are notorious blood-sucking ectoparasites and are completely dependent on blood-meals from hosts. In addition to the direct severe effects on health and productivity, ixodid ticks transmit various deadly diseases to humans and animals. Unlike rapidly feeding vessel-feeder hematophagous insects, the hard ticks feed on hosts for a long time (5-10 days or more), making a large blood pool beneath the skin. Tick's salivary glands produce a vast array of bio-molecules that modulate their complex and persistent feeding processes. However, the specific molecule that functions in the development and maintenance of a blood pool is yet to be identified. Recently, we have reported on longistatin, a 17.8-kDa protein with two functional EF-hand Ca(++)-binding domains, from the salivary glands of the disease vector, Haemaphysalis longicornis, that has been shown to be linked to blood-feeding processes. Here, we show that longistatin plays vital roles in the formation of a blood pool and in the acquisition of blood-meals. Data clearly revealed that post-transcriptional silencing of the longistatin-specific gene disrupted ticks' unique ability to create a blood pool, and they consequently failed to feed and replete on blood-meals from hosts. Longistatin completely hydrolyzed α, β and γ chains of fibrinogen and delayed fibrin clot formation. Longistatin was able to bind with fibrin meshwork, and activated fibrin clot-bound plasminogen into its active form plasmin, as comparable to that of tissue-type plasminogen activator (t-PA), and induced lysis of fibrin clot and platelet-rich thrombi. Plasminogen activation potentiality of longistatin was increased up to 4 times by soluble fibrin. Taken together, our results suggest that longistatin may exert potent functions both as a plasminogen activator and as an anticoagulant in the complex scenario of blood pool formation; the latter is critical to the feeding success and survival of ixodid ticks.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

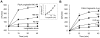

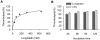
References
-
- Stark KR, James AA. Anticoagulant in vector arthropods. Parasitol Today. 1996;12:430–437. - PubMed
-
- Davie EW, Ratnoff OD. Waterfall sequence for intrinsic blood clotting. Science. 1964;145:1310–1312. - PubMed
-
- Riddel JP, Jr, Aouizerat BE, Miaskowski C, Lillicrap DP. Theories of blood coagulation. J Pediatr Oncol Nurs. 2007;24:123–131. - PubMed
-
- Byzova TV, Plow EF. Networking in the hemostatic system, integrin αIIbβ3 binds prothrobin and influences its activation. J Biol Chem. 1997;272:27183–27188. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

