TGF-β inducible early gene 1 regulates osteoclast differentiation and survival by mediating the NFATc1, AKT, and MEK/ERK signaling pathways
- PMID: 21423731
- PMCID: PMC3056664
- DOI: 10.1371/journal.pone.0017522
TGF-β inducible early gene 1 regulates osteoclast differentiation and survival by mediating the NFATc1, AKT, and MEK/ERK signaling pathways
Abstract
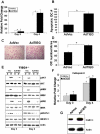
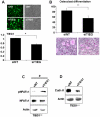
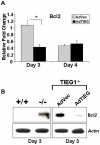
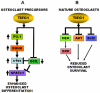
TGF-β Inducible Early Gene-1 (TIEG1) is a Krüppel-like transcription factor (KLF10) that was originally cloned from human osteoblasts as an early response gene to TGF-β treatment. As reported previously, TIEG1(-/-) mice have decreased cortical bone thickness and vertebral bone volume and have increased spacing between the trabeculae in the femoral head relative to wildtype controls. Here, we have investigated the role of TIEG1 in osteoclasts to further determine their potential role in mediating this phenotype. We have found that TIEG1(-/-) osteoclast precursors differentiated more slowly compared to wildtype precursors in vitro and high RANKL doses are able to overcome this defect. We also discovered that TIEG1(-/-) precursors exhibit defective RANKL-induced phosphorylation and accumulation of NFATc1 and the NFATc1 target gene DC-STAMP. Higher RANKL concentrations reversed defective NFATc1 signaling and restored differentiation. After differentiation, wildtype osteoclasts underwent apoptosis more quickly than TIEG1(-/-) osteoclasts. We observed increased AKT and MEK/ERK signaling pathway activation in TIEG1(-/-) osteoclasts, consistent with the roles of these kinases in promoting osteoclast survival. Adenoviral delivery of TIEG1 (AdTIEG1) to TIEG1(-/-) cells reversed the RANKL-induced NFATc1 signaling defect in TIEG1(-/-) precursors and eliminated the differentiation and apoptosis defects. Suppression of TIEG1 with siRNA in wildtype cells reduced differentiation and NFATc1 activation. Together, these data provide evidence that TIEG1 controls osteoclast differentiation by reducing NFATc1 pathway activation and reduces osteoclast survival by suppressing AKT and MEK/ERK signaling.
Conflict of interest statement
Figures




Similar articles
-
Siglec-15 regulates osteoclast differentiation by modulating RANKL-induced phosphatidylinositol 3-kinase/Akt and Erk pathways in association with signaling Adaptor DAP12.J Bone Miner Res. 2013 Dec;28(12):2463-75. doi: 10.1002/jbmr.1989. J Bone Miner Res. 2013. PMID: 23677868
-
Docosahexaenoic acid signaling attenuates the proliferation and differentiation of bone marrow-derived osteoclast precursors and promotes apoptosis in mature osteoclasts.Cell Signal. 2017 Jan;29:226-232. doi: 10.1016/j.cellsig.2016.11.007. Epub 2016 Nov 9. Cell Signal. 2017. PMID: 27836739
-
Molecular mechanism of the bifunctional role of lipopolysaccharide in osteoclastogenesis.J Biol Chem. 2009 May 1;284(18):12512-23. doi: 10.1074/jbc.M809789200. Epub 2009 Mar 3. J Biol Chem. 2009. PMID: 19258321 Free PMC article.
-
The role of NFATc1 in the progression and metastasis of prostate cancer: A review on the molecular mechanisms and signaling pathways.Cell Biol Int. 2023 Dec;47(12):1895-1904. doi: 10.1002/cbin.12094. Epub 2023 Oct 9. Cell Biol Int. 2023. PMID: 37814550 Review.
-
KLF10 as a Tumor Suppressor Gene and Its TGF-β Signaling.Cancers (Basel). 2018 May 25;10(6):161. doi: 10.3390/cancers10060161. Cancers (Basel). 2018. PMID: 29799499 Free PMC article. Review.
Cited by
-
TGF-βRI kinase activity mediates Emdogain-stimulated in vitro osteoclastogenesis.Clin Oral Investig. 2014 Jul;18(6):1639-46. doi: 10.1007/s00784-013-1129-6. Epub 2013 Nov 13. Clin Oral Investig. 2014. PMID: 24221580
-
Critical role of MKP-1 in lipopolysaccharide-induced osteoclast formation through CXCL1 and CXCL2.Cytokine. 2015 Jan;71(1):71-80. doi: 10.1016/j.cyto.2014.08.007. Epub 2014 Sep 27. Cytokine. 2015. PMID: 25261746 Free PMC article.
-
MKP-1 signaling events are required for early osteoclastogenesis in lineage defined progenitor populations by disrupting RANKL-induced NFATc1 nuclear translocation.Bone. 2014 Mar;60:16-25. doi: 10.1016/j.bone.2013.11.012. Epub 2013 Nov 20. Bone. 2014. PMID: 24269279 Free PMC article.
-
An in vitro 3D bone metastasis model by using a human bone tissue culture and human sex-related cancer cells.Oncotarget. 2016 Nov 22;7(47):76966-76983. doi: 10.18632/oncotarget.12763. Oncotarget. 2016. PMID: 27765913 Free PMC article.
-
Kruppel-like Factors in Skeletal Physiology and Pathologies.Int J Mol Sci. 2022 Dec 2;23(23):15174. doi: 10.3390/ijms232315174. Int J Mol Sci. 2022. PMID: 36499521 Free PMC article. Review.
References
-
- Jimi E, Aoki K, Saito H, D'Acquisto F, May MJ, et al. Selective inhibition of NF-kappa B blocks osteoclastogenesis and prevents inflammatory bone destruction in vivo. Nat Med. 2004;10:617–624. - PubMed
-
- Michigami T, Ihara-Watanabe M, Yamazaki M, Ozono K. Receptor activator of nuclear factor kappaB ligand (RANKL) is a key molecule of osteoclast formation for bone metastasis in a newly developed model of human neuroblastoma. Cancer Res. 2001;61:1637–1644. - PubMed
-
- Mansky KC, Sankar U, Han J, Ostrowski MC. Microphthalmia transcription factor is a target of the p38 MAPK pathway in response to receptor activator of NF-kappa B ligand signaling. J Biol Chem. 2002;277:11077–11083. - PubMed
-
- Ikeda F, Matsubara T, Tsurukai T, Hata K, Nishimura R, et al. JNK/c-Jun signaling mediates an anti-apoptotic effect of RANKL in osteoclasts. J Bone Miner Res. 2008;23:907–914. - PubMed
-
- Gingery A, Bradley E, Shaw A, Oursler MJ. Phosphatidylinositol 3-kinase coordinately activates the MEK/ERK and AKT/NFkappaB pathways to maintain osteoclast survival. J Cell Biochem. 2003;89:165–179. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

