Central Arterial Aging and Angiotensin II Signaling
- PMID: 21423831
- PMCID: PMC3058527
- DOI: 10.2174/157340210793611668
Central Arterial Aging and Angiotensin II Signaling
Abstract
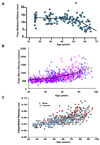
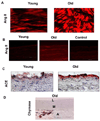
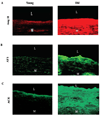
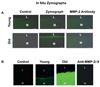
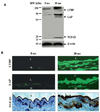
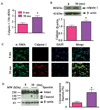
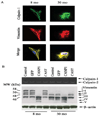
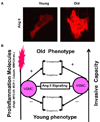
Arterial remodeling over time is a cornerstone of normal systemic aging. The age-associated arterial structural and functional changes in the intima, the media, and the adventitia are closely linked to angiotensin II (Ang II) signaling. A growing line of evidence indicates that essential elements of Ang II signaling, which encompasses milk fat globule epidermal growth factor-8, calpain-1, transforming growth factor-β1, matrix metalloproteinase-2/9, monocyte chemoattractant protein-1, nicotinamide adenine dinucleotide phosphate-oxidase, and reactive oxygen species, are upregulated within the central arterial wall in rats, nonhuman primates, and humans during aging. In vitro studies show that the elevation of Ang II signaling induces the accumulation of collagen and advanced glycated end-products, the degradation of elastin, and the increased cell cycle disorder, invasion, and hypertrophy of endothelial and vascular smooth muscle cells. Further, in vivo studies demonstrate that increased Ang II signaling accelerates arterial aging. Conversely, attenuating Ang II signaling via an inhibition of angiotensin conversing enzyme or a blockade of AT1 activation retards age-associated arterial remodeling. This review attempts to integrate complex facts of Ang II signaling within the aged central arterial wall and may shed light on new therapeutic targets for arterial aging.
Conflict of interest statement
None
Figures




References
-
- Li Z, Cheng H, Lederer WJ, et al. Enhanced proliferation and migration and altered cytoskeletal proteins in early passage smooth muscle cells from young and old rat aortic explants. Exp Mol Pathol. 1997;64:1–11. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
