Seven-coordinate anion complex with a tren-based urea: binding discrepancy of hydrogen sulfate in solid and solution states
- PMID: 21424002
- PMCID: PMC3306551
- DOI: 10.1039/c1ob05052d
Seven-coordinate anion complex with a tren-based urea: binding discrepancy of hydrogen sulfate in solid and solution states
Abstract
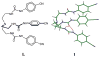
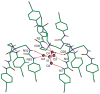
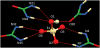
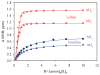
Structural characterization of a hydrogen sulfate complex with a tren-based urea suggests that the anion is coordinated with six NH···O bonds (d(N···O) = 2.857 (3) to 3.092 (3) Å) and one OH···O bond (d(O···O) = 2.57 (2) Å) from three receptors; however, in solution the anion is bound within the pseudo-cavity of one receptor.
Figures
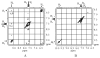
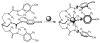
References
-
- Antonisse MMG, Reinhoudt DN. Chem Commun. 1998:443–448.
- Atwood JL, Holman KT, Steed JW. Chem Commun. 1996:1401–1407.
- Gale PA. Coord Chem Rev. 2003;240:191–221.
- Cormode DP, Murray SS, Cowley AR, Beer PD. Dalton Trans. 2006:5135–5140. - PubMed
-
- Pflugrath JW, Quiocho FA. Nature. 1985;314:257–260. - PubMed
-
- Moyer BA, Delmau LH, Fowler CJ, Ruas A, Bostick DA, Sessler JL, Katayev E, Pantos GD, Llinares JM, Hossain MA, Kang SO, Bowman-James K. Adv Inorg Chem. 2007;59:175–204.
-
- Kang SO, Hossain MA, Powell D, Bowman-James K. Chem Commun. 2005:328–330. - PubMed
- Mani G, Guchhait T, Kumar R, Kumar S. Org Lett. 2010;12:3910–3913. - PubMed
- Mendy JS, Pilate M, Horne T, Day VW, Hossain MA. Chem Commun. 2010;46:6084–6086. - PMC - PubMed
- Saeed MA, Fronczek FR, Powell DR, Hossain MA. Tetrahedron Lett. 2010;51:4233–4236. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

