Rate-limiting domain and loop motions in arginine kinase
- PMID: 21425868
- PMCID: PMC3091953
- DOI: 10.1021/bi101664u
Rate-limiting domain and loop motions in arginine kinase
Abstract
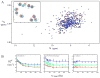
Arginine kinase catalyzes the reversible transfer of a phosphoryl group between ATP and arginine. It is the arthropod homologue of creatine kinase, buffering cellular ATP levels. Crystal structures of arginine kinase, in substrate-free and substrate-bound forms, have revealed large conformational changes associated with the catalytic cycle. Recent nuclear magnetic resonance identified movements of the N-terminal domain and a loop comprising residues I182--G209 with conformational exchange rates in the substrate-free enzyme similar to the turnover rate. Here, to understand whether these motions might be rate-limiting, we determined activation barriers for both the intrinsic dynamics and enzyme turnover using measurements over a temperature range of 15-30 °C. (15)N transverse relaxation dispersion yields activation barriers of 46 ± 8 and 34 ± 12 kJ/mol for the N-terminal domain and I182--G209 loop, respectively. An activation barrier of 34 ± 13 kJ/mol was obtained for enzyme turnover from steady-state kinetics. The similarity between the activation barriers is indeed consistent with turnover being limited by backbone conformational dynamics and pinpoints the locations of potentially rate-limiting motions.
Figures



References
-
- Ellington WR. Evolution and Physiological Roles of Phosphagen Systems. Annual Review of Physiology. 2001;63:289–325. - PubMed
-
- Davulcu O, Clark SA, Chapman MS, Skalicky JJ. Main chain (1)H, (13)C, and (15)N resonance assignments of the 42-kDa enzyme arginine kinase. J Biomol NMR. 2005;32:178. - PubMed
-
- Yousef MS, Fabiola F, Gattis J, Somasundaram T, Chapman MS. Refinement of Arginine Kinase Transition State Analogue Complex at 1.2 Å resolution; mechanistic insights. Acta Crystallographica Section D: Biological Crystallography. 2002;58:2009–2017. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

