S-glutathionyl-(chloro)hydroquinone reductases: a new class of glutathione transferases functioning as oxidoreductases
- PMID: 21425927
- PMCID: PMC3233476
- DOI: 10.3109/03602532.2011.552909
S-glutathionyl-(chloro)hydroquinone reductases: a new class of glutathione transferases functioning as oxidoreductases
Abstract
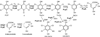
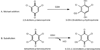
Glutathione transferases (GSTs) are best known for transferring glutathione (GSH) to hydrophobic organic compounds, making the conjugates more soluble. However, the omega-class GSTs of animals and the lambda-class GSTs and dehydroascorbate reductases (DHARs) of plants have little or no activity for GSH transfer. Instead, they catalyze GSH-dependent oxidoreductions. The lambda-class GSTs reduce disulfide bonds, the DHARs reduce the disulfide bonds and dehydroascorbate, and the omega-class GSTs can reduce more substrates, including disulfide bonds, dehydroascorbate, and dimethylarsinate. Glutathionyl-(chloro)hydroquinone reductases (GS-HQRs) are the newest class of GSTs that mainly catalyze oxidoreductions. Besides the activities of the other three classes, GS-HQRs also reduce GS-hydroquinones, including GS-trichloro-p-hydroquinone, GS-dichloro-p-hydroquinone, GS-2-hydroxy-p-hydroquinone, and GS-p-hydroquinone. They are conserved and widely distributed in bacteria, fungi, protozoa, and plants, but not in animals. The four classes are phylogenetically more related to each other than to other GSTs, and they share a Cys-Pro motif at the GSH-binding site. Hydroquinones are metabolic intermediates of certain aromatic compounds. They can be auto-oxidized by O(2) to benzoquinones, which spontaneously react with GSH to form GS-hydroquinones via Michael's addition. GS-HQRs are expected to channel GS-hydroquinones, formed spontaneously or enzymatically, back to hydroquinones. When the released hydroquinones are intermediates of metabolic pathways, GS-HQRs play a maintenance role for the pathways. Further, the common presence of GS-HQRs in plants, green algae, cyanobacteria, and halobacteria suggest a beneficial role in the light-using organisms.
Figures





References
-
- Allocati N, Federici L, Masulli M, Di Ilio C. Glutathione transferases in bacteria. FEBS J. 2009;276:58–75. - PubMed
-
- Allocati N, Federici L, Masulli M, Favaloro B, Di Ilio C. Cysteine 10 is critical for the activity of Ochrobactrum anthropi glutathione transferase and its mutation to alanine causes the preferential binding of glutathione to the H-site. Proteins. 2008;71:16–23. - PubMed
-
- Anandarajah K, Kiefer PMJ, Donohoe BS, Copley SD. Recruitment of a double bond isomerase to serve as a reductive dehalogenase during biodegradation of pentachlorophenol. Biochemistry. 2000;39:5303–5311. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
