PGE(2) -EP(2) signalling in endothelium is activated by haemodynamic stress and induces cerebral aneurysm through an amplifying loop via NF-κB
- PMID: 21426319
- PMCID: PMC3144537
- DOI: 10.1111/j.1476-5381.2011.01358.x
PGE(2) -EP(2) signalling in endothelium is activated by haemodynamic stress and induces cerebral aneurysm through an amplifying loop via NF-κB
Abstract
Background and purpose: Cerebral aneurysm is a frequent cerebrovascular event and a major cause of fatal subarachnoid haemorrhage, but there is no medical treatment for this condition. Haemodynamic stress and, recently, chronic inflammation have been proposed as major causes of cerebral aneurysm. Nevertheless, links between haemodynamic stress and chronic inflammation remain ill-defined, and to clarify such links, we evaluated the effects of prostaglandin E(2) (PGE(2) ), a mediator of inflammation, on the formation of cerebral aneurysms.
Experimental approach: Expression of COX and prostaglandin E synthase (PGES) and PGE receptors were examined in human and rodent cerebral aneurysm. The incidence, size and inflammation of cerebral aneurysms were evaluated in rats treated with COX-2 inhibitors and mice lacking each prostaglandin receptor. Effects of shear stress and PGE receptor signalling on expression of pro-inflammatory molecules were studied in primary cultures of human endothelial cells (ECs).
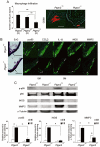
Key results: COX-2, microsomal PGES-1 and prostaglandin E receptor 2 (EP(2) ) were induced in ECs in the walls of cerebral aneurysms. Shear stress applied to primary ECs induced COX-2 and EP(2) . Inhibition or loss of COX-2 or EP(2) in vivo attenuated each other's expression, suppressed nuclear factor κB (NF-κB)-mediated chronic inflammation and reduced incidence of cerebral aneurysm. EP(2) stimulation in primary ECs induced NF-κB activation and expression of the chemokine (C-C motif) ligand 2, essential for cerebral aneurysm.
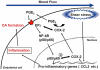
Conclusions and implications: These results suggest that shear stress activated PGE(2) -EP(2) pathway in ECs and amplified chronic inflammation via NF-κB. We propose EP(2) as a therapeutic target in cerebral aneurysm.
© 2011 The Authors. British Journal of Pharmacology © 2011 The British Pharmacological Society.
Figures


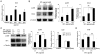
References
-
- Alnaes MS, Isaksen J, Mardal KA, Romner B, Morgan MK, Ingebrigtsen T. Computation of hemodynamics in the circle of Willis. Stroke. 2007;38:2500–2505. - PubMed
-
- Aoki T, Nishimura M. Targeting chronic inflammation in cerebral aneurysms: focusing on NF-κB as a putative target of medical therapy. Expert Opin Ther Targets. 2010;14:265–273. - PubMed
-
- Aoki T, Kataoka H, Morimoto M, Nozaki K, Hashimoto N. Macrophage-derived matrix metalloproteinase-2 and -9 promote the progression of cerebral aneurysms in rats. Stroke. 2007a;38:162–169. - PubMed
-
- Aoki T, Kataoka H, Shimamura M, Nakagami H, Wakayama K, Moriwaki T, et al. NF-κB is a key mediator of cerebral aneurysm formation. Circulation. 2007b;116:2830–2840. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

