SB-656933, a novel CXCR2 selective antagonist, inhibits ex vivo neutrophil activation and ozone-induced airway inflammation in humans
- PMID: 21426372
- PMCID: PMC3162658
- DOI: 10.1111/j.1365-2125.2011.03968.x
SB-656933, a novel CXCR2 selective antagonist, inhibits ex vivo neutrophil activation and ozone-induced airway inflammation in humans
Abstract
What is already known about this subject: Receptor antagonists that block the binding of chemokines such as CXCL8 (IL-8) are effective in animals models of neutrophil-mediated inflammation. It has been hypothesized that selective inhibition of neutrophil trafficking and activation may be a useful adjunct for the treatment of inflammatory airway diseases such as chronic obstructive pulmonary disease or cystic fibrosis. A CXCR1/2 receptor antagonist has shown activity in an ozone challenge model in humans.
What this study adds: SB-656933, a selective CXCR2 antagonist, is safe and well-tolerated at single doses and is shown to inhibit agonist (CXCL1)-mediated expression of the CD11b on peripheral blood neutrophils as well as ozone-induced airway neutrophilia in healthy subjects.
Aims: To determine the safety and tolerability of a novel selective CXCR2 antagonist and assess its pharmacodynamic effects using measures of neutrophil activation and function, including CD11b expression in whole blood and ozone-induced airway inflammation in healthy subjects.
Methods: Flow cytometric determination of ex vivo CXCL1-induced CD11b expression on peripheral blood neutrophils was performed following single dose oral administration of SB-656933 (dose range 2-1100 mg). A subsequent randomized study (placebo, 50 mg and 150 mg) was performed to explore the dose-response for ozone-induced airway inflammation, as measured by sputum biomarkers.
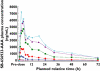
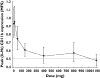
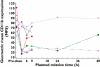
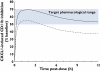
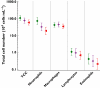
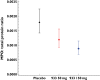
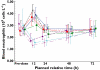
Results: Oral administration of SB-656933 resulted in significant inhibition of CXCL1-induced CD11b expression on peripheral blood neutrophils at single doses greater than or equal to 50 mg. Maximum inhibition (70%) relative to placebo was observed following administration of SB-656933 400 mg (95% CI 60%, 77%). This was sustained up to a dose of 1100 mg. Single doses of SB-656933 reduced ozone-induced airway inflammation in a dose-dependent manner. Relative to placebo, there were 55% (95% CI 20%, 75%) and 74% (95% CI 55%, 85%) fewer neutrophils in the sputum of subjects after a single dose of 50 mg or 150 mg, respectively. There was a corresponding reduction in myeloperoxidase concentrations in the sputum supernatant of 32.8% (95% CI 9.2, 50.3) and 50.5% (95% CI 33.3, 63.3). SB-656933 was safe and well-tolerated at all doses.
Conclusions: SB-656933 is a CXCR2 antagonist that demonstrates dose-dependent effects on neutrophil activation and recruitment within a well-tolerated dose range. These data suggest that SB-656933 may be an effective agent in neutrophil-predominant diseases.
© 2011 The Authors. British Journal of Clinical Pharmacology © 2011 The British Pharmacological Society.
Figures
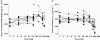
References
-
- Quint JK, Wedzicha JA. The neutrophil in chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2007;119:1065–71. - PubMed
-
- Tirouvanziam R. Neutrophilic inflammation as a major determinant in the progression of cystic fibrosis. Drug News Perspect. 2006;19:609–14. - PubMed
-
- Wang Q, Doerschuk CM, Mizgerd JP. Neutrophils in innate immunity. Semin Respir Crit Care Med. 2004;25:33–41. - PubMed
-
- Tsai HH, Frost E, To V, Robinson S, ffrench-Constant C, Geertman R, Ransohoff RM, Miller RH. The chemokine receptor CXCR2 controls positioning of oligodendrocyte precursors in developing spinal cord by arresting their migration. Cell. 2002;110:373–83. - PubMed
-
- Stillie R, Farooq SM, Gordon JR, Stadnyk AW. The functional significance behind expressing two IL-8 receptor types on PMN. J Leukoc Biol. 2009;86:529–43. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

