Deletion of the Slo3 gene abolishes alkalization-activated K+ current in mouse spermatozoa
- PMID: 21427226
- PMCID: PMC3078394
- DOI: 10.1073/pnas.1100240108
Deletion of the Slo3 gene abolishes alkalization-activated K+ current in mouse spermatozoa
Abstract
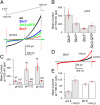
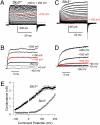
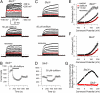
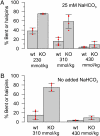
Mouse spermatozoa express a pH-dependent K(+) current (KSper) thought to be composed of subunits encoded by the Slo3 gene. However, the equivalence of KSper and Slo3-dependent current remains uncertain, because heterologous expression of Slo3 results in currents that are less effectively activated by alkalization than are native KSper currents. Here, we show that genetic deletion of Slo3 abolishes all pH-dependent K(+) current at physiological membrane potentials in corpus epididymal sperm. A residual pH-dependent outward current (I(Kres)) is observed in Slo3(-/-) sperm at potentials of >0 mV. Differential inhibition of KSper/Slo3 and I(Kres) by clofilium reveals that the amplitude of I(Kres) is similar in both wild-type (wt) and Slo3(-/-) sperm. The properties of I(Kres) suggest that it likely represents outward monovalent cation flux through CatSper channels. Thus, KSper/Slo3 may account for essentially all mouse sperm K(+) current and is the sole pH-dependent K(+) conductance in these sperm. With physiological ionic gradients, alkalization depolarizes Slo3(-/-) spermatozoa, presumably from CatSper activation, in contrast to Slo3/KSper-mediated hyperpolarization in wt sperm. Slo3(-/-) male mice are infertile, but Slo3(-/-) sperm exhibit some fertility within in vitro fertilization assays. Slo3(-/-) sperm exhibit a higher incidence of morphological abnormalities accentuated by hypotonic challenge and also exhibit deficits in motility in the absence of bicarbonate, revealing a role of KSper under unstimulated conditions. Together, these results show that KSper/Slo3 is the primary spermatozoan K(+) current, that KSper may play a critical role in acquisition of normal morphology and sperm motility when faced with hyperosmotic challenges, and that Slo3 is critical for fertility.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Visconti PE, Kopf GS. Regulation of protein phosphorylation during sperm capacitation. Biol Reprod. 1998;59:1–6. - PubMed
-
- Suarez SS. Control of hyperactivation in sperm. Hum Reprod Update. 2008;14:647–657. - PubMed
-
- Nixon B, et al. Elucidation of the signaling pathways that underpin capacitation-associated surface phosphotyrosine expression in mouse spermatozoa. J Cell Physiol. 2010;224:71–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

