Physiological role and regulation of iodothyronine deiodinases: a 2011 update
- PMID: 21427525
- PMCID: PMC3687787
- DOI: 10.1007/BF03347465
Physiological role and regulation of iodothyronine deiodinases: a 2011 update
Abstract
T4 is a prohormone secreted by the thyroid. T4 has a long half life in circulation and it is tightly regulated to remain constant in a variety of circumstances. However, the availability of iodothyronine selenodeiodinases allow both the initiation or the cessation of thyroid hormone action and can result in surprisingly acute changes in the intracellular concentration of the active hormone T3, in a tissue- specific and chronologically-determined fashion, in spite of the constant circulating levels of the prohormone. This fine-tuning of thyroid hormone signaling is becoming widely appreciated in the context of situations where the rapid modifications in intracellular T3 concentrations are necessary for developmental changes or tissue repair. Given the increasing availability of genetic models of deiodinase deficiency, new insights into the role of these important enzymes are being recognized. In this review, we have incorporated new information regarding the special role played by these enzymes into our current knowledge of thyroid physiology, emphasizing the clinical significance of these new insights.
Figures
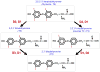


References
-
- Berry MJ, Maia AL, Kieffer JD, Harney JW. Larsen PR Substitution of cysteine for selenocysteine in type I iodothyronine deiodinase reduces the catalytic efficiency of the protein but enhances its translation. Endocrinology. 1992;131:1848–1852. - PubMed
-
- Berry MJ, Banu L, Chen YY, et al. Recognition of UGA as a selenocysteine codon in type I deiodinase requires sequences in the 3’ untranslated region. Nature. 1991;353:273–276. - PubMed
-
- Berry MJ. Knowing when not to stop. Nat Struct Mol Biol. 2005;12:389–390. - PubMed
-
- Bianco AC, Salvatore D, Gereben B, Berry MJ. Larsen PR Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev. 2002;23:38–89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

