Grb2 regulates B-cell maturation, B-cell memory responses and inhibits B-cell Ca2+ signalling
- PMID: 21427701
- PMCID: PMC3102284
- DOI: 10.1038/emboj.2011.74
Grb2 regulates B-cell maturation, B-cell memory responses and inhibits B-cell Ca2+ signalling
Abstract
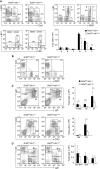
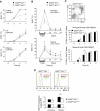
Grb2 is a ubiquitously expressed adaptor protein, which activates Ras and MAP kinases in growth factor receptor signalling, while in B-cell receptor (BCR) signalling this role is controversial. In B cell lines it was shown that Grb2 can inhibit BCR-induced Ca(2+) signalling. Nonetheless, the physiological role of Grb2 in primary B cells is still unknown. We generated a B-cell-specific Grb2-deficient mouse line, which had a severe reduction of mature follicular B cells in the periphery due to a differentiation block and decreased B-cell survival. Moreover, we found several changes in important signalling pathways: enhanced BCR-induced Ca(2+) signalling, alterations in mitogen-activated protein kinase activation patterns and strongly impaired Akt activation, the latter pointing towards a defect in PI3K signalling. Interestingly, B-cell-specific Grb2-deficient mice showed impaired IgG and B-cell memory responses, and impaired germinal centre formation. Thus, Grb2-dependent signalling pathways are crucial for lymphocyte differentiation processes, as well as for control of secondary humoral immune responses.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



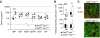


References
-
- Buday L, Downward J (1993) Epidermal growth factor regulates p21ras through the formation of a complex of receptor, Grb2 adapter protein, and Sos nucleotide exchange factor. Cell 73: 611–620 - PubMed
-
- Cheng AM, Saxton TM, Sakai R, Kulkarni S, Mbamalu G, Vogel W, Tortorice CG, Cardiff RD, Cross JC, Muller WJ, Pawson T (1998) Mammalian Grb2 regulates multiple steps in embryonic development and malignant transformation. Cell 95: 793–803 - PubMed
-
- Coughlin JJ, Stang SL, Dower NA, Stone JC (2005) RasGRP1 and RasGRP3 regulate B cell proliferation by facilitating B cell receptor-Ras signaling. J Immunol 175: 7179–7184 - PubMed
-
- Egan SE, Giddings BW, Brooks MW, Buday L, Sizeland AM, Weinberg RA (1993) Association of Sos Ras exchange protein with Grb2 is implicated in tyrosine kinase signal transduction and transformation. Nature 363: 45–51 - PubMed
-
- Engels N, Konig LM, Heemann C, Lutz J, Tsubata T, Griep S, Schrader V, Wienands J (2009) Recruitment of the cytoplasmic adaptor Grb2 to surface IgG and IgE provides antigen receptor-intrinsic costimulation to class-switched B cells. Nat Immunol 10: 1018–1025 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

