Phosphoramidite gold(I)-catalyzed diastereo- and enantioselective synthesis of 3,4-substituted pyrrolidines
- PMID: 21428409
- PMCID: PMC3071892
- DOI: 10.1021/ja200084a
Phosphoramidite gold(I)-catalyzed diastereo- and enantioselective synthesis of 3,4-substituted pyrrolidines
Abstract
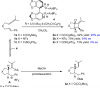
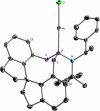
In this article the utility of phosphoramidite ligands in enantioselective Au(I) catalysis was explored in the development of highly diastereo- and enantioselective Au(I)-catalyzed cycloadditions of allenenes. A Au(I)-catalyzed synthesis of 3,4-disubstituted pyrrolidines and γ-lactams is described. This reaction proceeds through the enantioselective Au(I)-catalyzed cyclization of allenenes to form a carbocationic intermediate that is trapped by an exogenous nucleophile, resulting in the highly diastereoselective construction of three contiguous stereogenic centers. A computational study (DFT) was also performed to gain some insight into the underlying mechanisms of these cycloadditions. The utility of this new methodology was demonstrated through the formal synthesis of (-)-isocynometrine.
© 2011 American Chemical Society
Figures




References
-
- Cordell GA, editor. The Alkaloids: Chemistry and Biology. Vol. 54. Academic Press; San Diego, CA: 2000.
- O'Hagan D. Nat. Prod. Rep. 2000;17:435. - PubMed
- Schomaker JM, Bhattacherjee JY, Borhan B. J. Am. Chem. Soc. 2007;129:1996. and references cited therein. - PubMed
- Hanessian S, Yun H, Tintelnot-Blomley M. J. Org. Chem. 2005;70:6746. - PubMed
-
-
For selected recent examples of enantioselective synthesis of pyrrolidines see: Zhang Z, Bender CF, Widenhoefer RA. Org. Lett. 2007;9:2887. Hamilton GL, Kang EJ, Mba M, Toste FD. Science. 2007;317:496. LaLonde RL, Sherry BD, Kang EJ, Toste FD. J. Am. Chem. Soc. 2007;129:2452. Shen X, Buchwald SL. Angew. Chem., Int. Ed. 2010;49:564. Mai DN, Wolfe JP. J. Am. Chem. Soc. 2010;132:12157.
-
-
- Cabrera S, Arrayas RG, Carretero JC. J. Am. Chem. Soc. 2005;127:16394. - PubMed
- Saito S, Tsibogo T, Kobayashi S. J. Am. Chem. Soc. 2007;129:5364. - PubMed
- Zeng W, Chen G-Y, Zhou Y-G, Li Y-X. J. Am. Chem. Soc. 2007;129:750. - PubMed
- Najera C, Retamosa MG, Sansano JM. Angew. Chem,. Int. Ed. 2008;47:6055. - PubMed
- Wang C-J, Liang G, Xue Z-Y, Gao F. J. Am. Chem. Soc. 2008;130:17250. - PubMed
- Parsons AT, Smith AG, Neel AJ, Johnson JS. J. Am. Chem. Soc. 2010;132:9688. - PubMed
-
- Cao P, Zhang X. Angew. Chem,. Int. Ed. 2000;39:4104. - PubMed
- Lei A, Waldrich JP, He M, Zhang X. Angew. Chem., Int. Ed. 2002;41:4527.
- Chakrapani H, Liu C, Widenhoefer RA. Org. Lett. 2003;5:157. - PubMed
- Jang H-Y, Hughes FW, Gong H, Zhnag J, Brodbelt JS, Krische MJ. J. Am. Chem. Soc. 2005;127:6174. - PubMed
- Xu W, Kong A, Lu X. J. Org. Chem. 2006;71:3854. - PubMed
- Rhee JU, Krische MJ. J. Am. Chem. Soc. 2006;128:10674. - PubMed
- Nicolaou KC, Li A, Ellery SP, Edmonds DJ. Angew. Chem., Int. Ed. 2009;48:6293. - PMC - PubMed
-
-
3,4-Substituted pyrrolidines comprise a structural motif commonly found in natural products and biologically active molecules: Farwick A, Helmchen G. Org. Lett. 2010;12:1108. Ohki S, Nagasaka T, Matsuda H, Ozawa N, Hamaguchi F. Chem. Pharm. Bull. 1986;34:3606. Okazaki Y, Ishizuka A, Ishihara A, Nishioka T, Iwamura H. J. Org. Chem. 2007;72:3830. Yu S, Qin D, Shangary JC, Wang G, Ding K, McEachern D, Qiu S, Nikolovska-Coleska Z, Miller R, Kang S, Yang D, Wang S. J. Med. Chem. 2009;52:7970. Ali A, Ahmad VU, Ziemer B, Liebscher J. Tetrahedron: Asymmetry. 2000;11:4365. Clayden J, Knowles FE, Menet CJ. Tetrahedron Lett. 2003;44:3397. Cook GR, Sun L. Org. Lett. 2004;6:2481. Lian G-Y, Yu J-D, Yang H-F, Lin F, Gao Q, Zhang D-W. Chem. Lett. 2007;36:408. Han X, Luo J, Liu C, Lu Y. Chem. Commun. 2009:2044.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

