Palladium(II)-catalyzed alkene functionalization via nucleopalladation: stereochemical pathways and enantioselective catalytic applications
- PMID: 21428440
- PMCID: PMC3076534
- DOI: 10.1021/cr100371y
Palladium(II)-catalyzed alkene functionalization via nucleopalladation: stereochemical pathways and enantioselective catalytic applications
Figures






























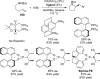

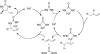

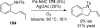
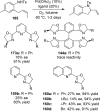









References
-
- Smidt J, Hafner W, Jira R, Sedlmeier S, Sieber R, Rüttinger R, Kojer H. Angew. Chem. 1959;71:176.
- Smidt J, Hafner W, Jira R, Sieber R, Sedlmeier S, Sabel A. Angew. Chem., Int. Ed. 1962;1:80.
- Jira R. Angew. Chem. Int. Ed. 2009;48:9034. - PubMed
-
-
For recent reviews of the Wacker process, see: Takacs JM, Jiang X. Curr. Org. Chem. 2003;7:369. Cornell CN, Sigman MS. Inorg. Chem. 2007;46:1903. Muzart J. Tetrahedron. 2007;63:7505.
-
-
- Keith JA, Henry PM. Angew. Chem., Int. Ed. 2009;48:9038. - PubMed
-
- Phillips FC. Am. Chem. J. 1894;16:255.
-
-
For reviews, see: Henry PM. Palladium Catalyzed Oxidation of Hydrocarbons. Vol. 2. Metal Complexes; D. Reidel Publishing Co.; Dordrecht: 1980. Bäckvall JE. Acc. Chem. Res. 1983;16:335. Negishi E, editor. Handbook of Organopalladium Chemistry for Organic Synthesis. Vol. 2. Wiley; New York: 2002. Jira R. In: Applied Homogeneous Catalysis with Organometallic Compounds. Weinheim. 2nd ed. Cornils B, Herrmann WA, editors. Wiley-VCH; 2002. p. 386. Sigman MS, Schultz MJ. Org. Biomol. Chem. 2004;2:2551. Stoltz BM. Chem. Lett. 2004;33:362. Tietze LF, Ila H, Bell HP. Chem. Rev. 2004;104:3453. Minatti A, Muniz K. Chem. Soc. Rev. 2007;36:1142. Kotov V, Scarborough CC, Stahl SS. Inorg. Chem. 2007;46:1910. Beccalli EM, Broggini G, Martinelli M, Sottocornola S. Chem. Rev. 2007;107:5318. Jensen KH, Sigman MS. Org. Biomol. Chem. 2008;6:4083. Gligorich KM, Sigman MS. Chem. Commun. 2009:3854.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

